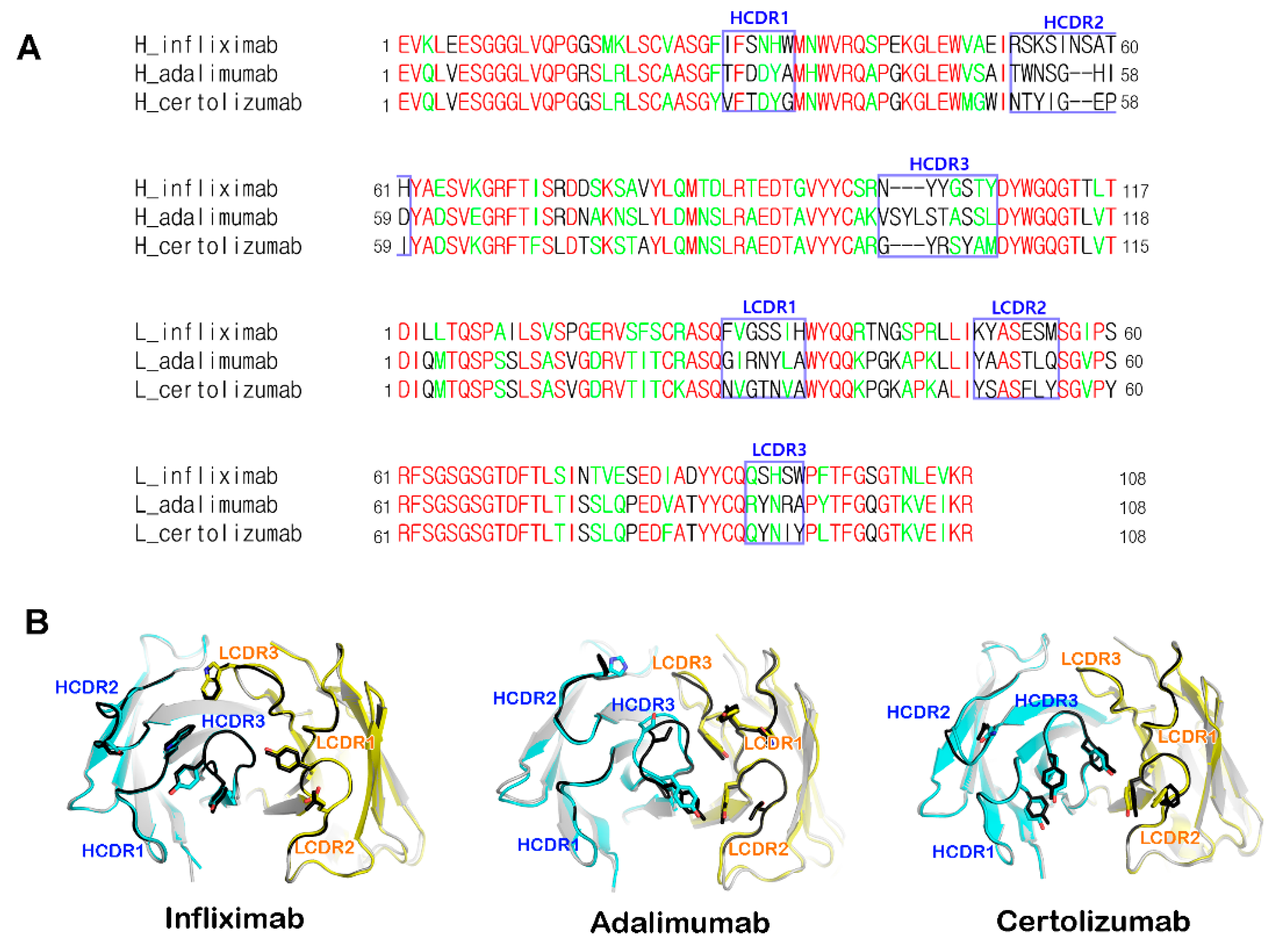
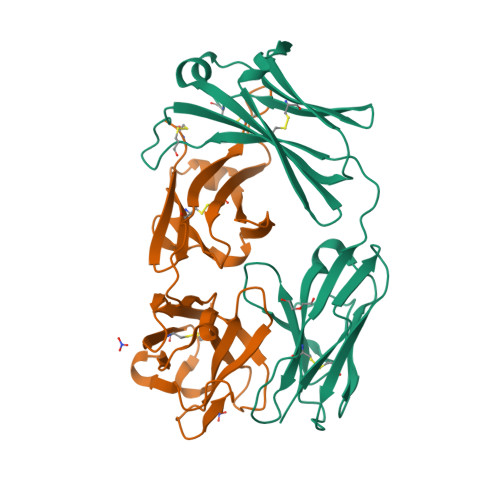
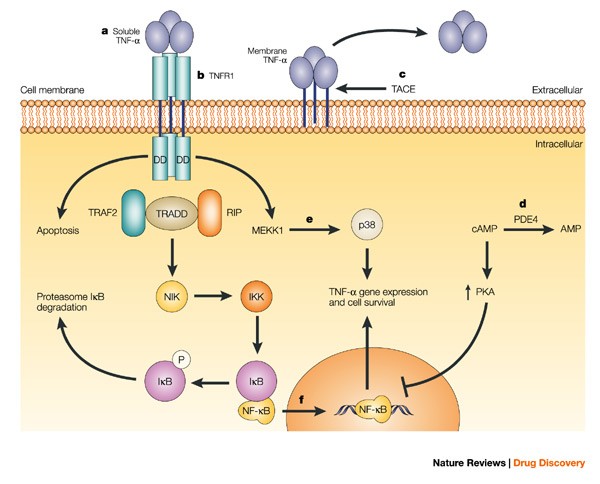
IJMS | Free Full-Text | Structural Biology of the TNFα Antagonists Used in the Treatment of Rheumatoid Arthritis

Increased versus conventional adalimumab dose interval for patients with Crohn's disease in stable remission (LADI): a pragmatic, open-label, non-inferiority, randomised controlled trial - The Lancet Gastroenterology & Hepatology
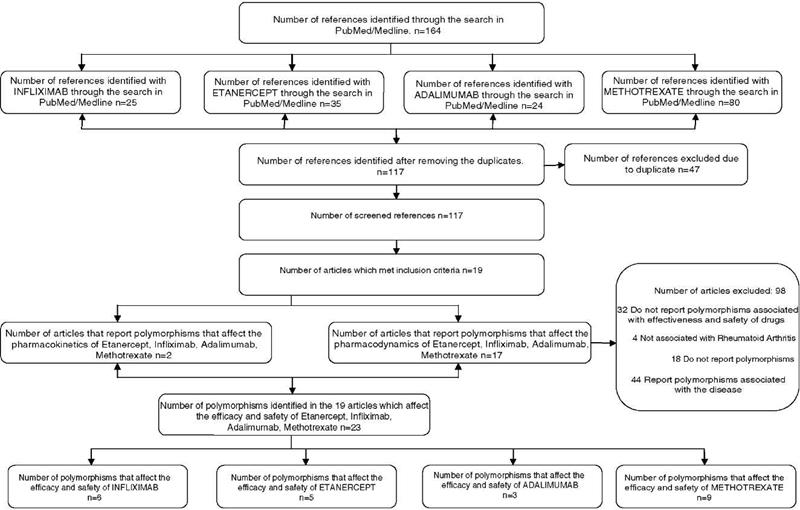
Pharmacogenomics of etanercept, infliximab, adalimumab and methotrexate in rheumatoid arthritis. A structured review

Comparison of consistency and complementarity of reporting biosimilar quality attributes between regulatory and scientific communities: An adalimumab case study - ScienceDirect
Patient perspectives of successful adalimumab biosimilar transitioning in Crohn's disease: an interview study

Implications for sequencing of biologic therapy and choice of second anti‐TNF in patients with inflammatory bowel disease: results from the IMmunogenicity to Second Anti‐TNF therapy (IMSAT) therapeutic drug monitoring study - Chanchlani -
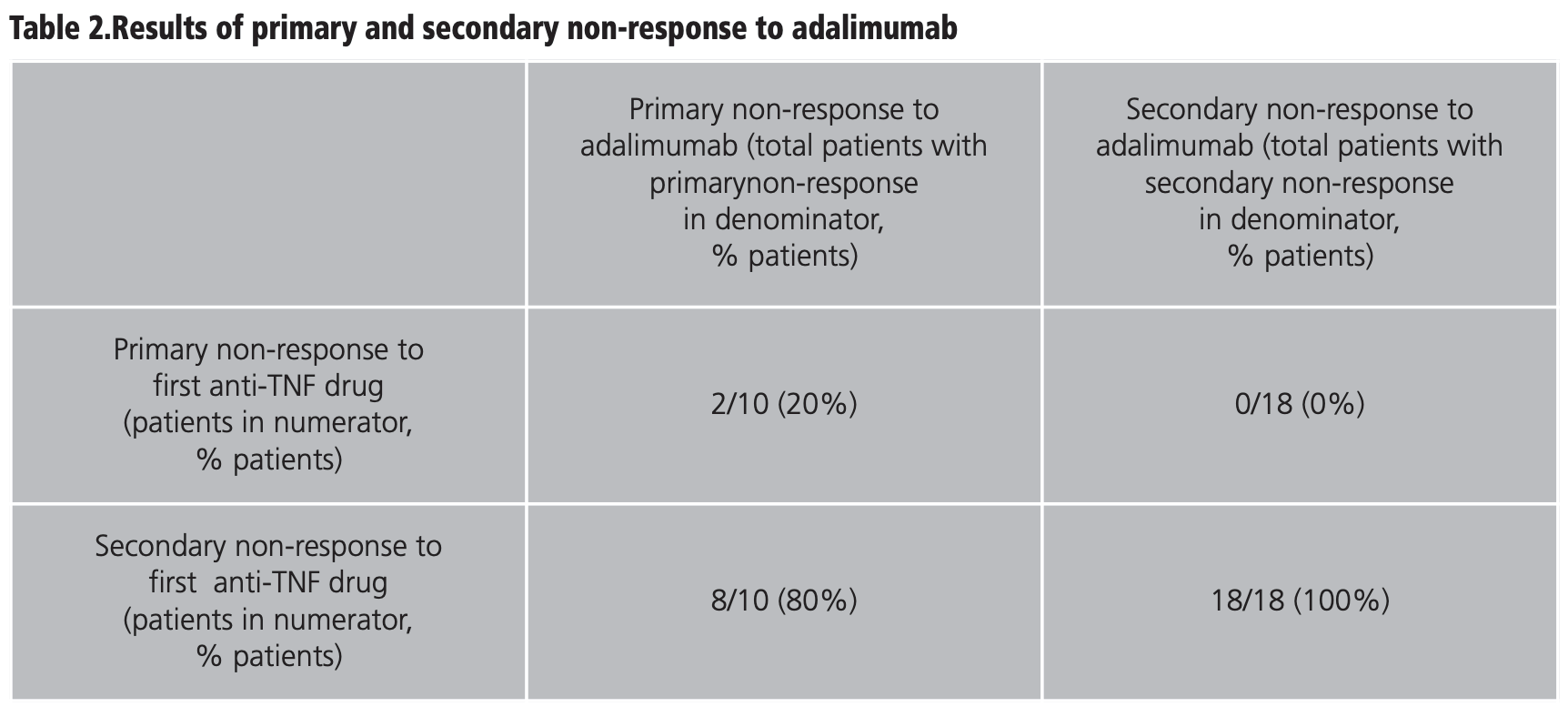
Influence of first-line anti-TNF therapy on the effectiveness of adalimumab in ulcerative colitis: long-term clinical practice data - ILAPHAR | Revista de la OFIL

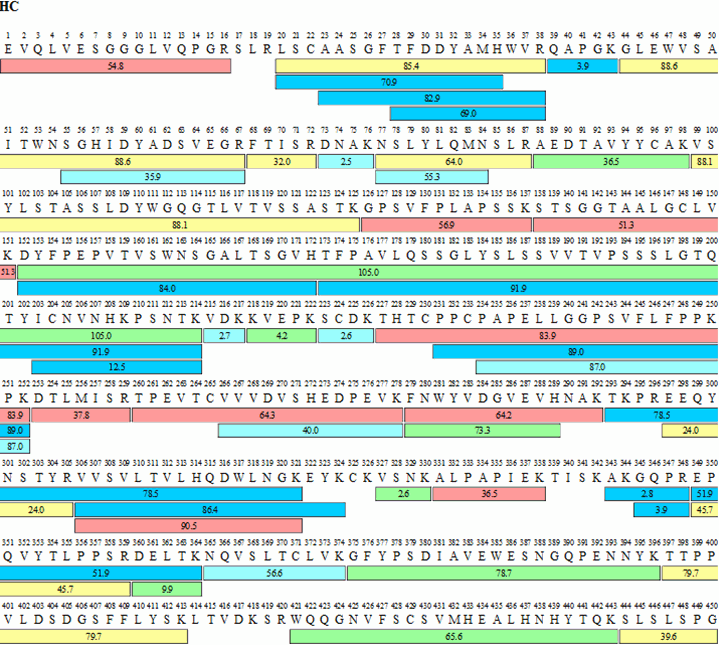
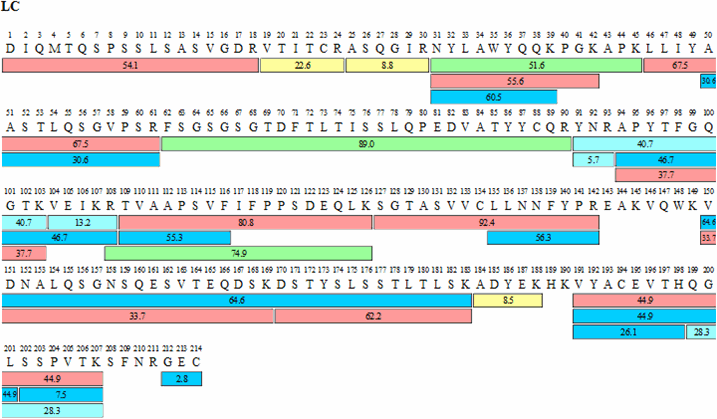
Amino acid sequences of adalimumab variable heavy (VH) and light chain... | Download Scientific Diagram

Figure 5 from Molecular Basis for the Neutralization of Tumor Necrosis Factor α by Certolizumab Pegol in the Treatment of Inflammatory Autoimmune Diseases | Semantic Scholar

The amino acid sequences of abatacept, alefacept, etanercept, and H... | Download Scientific Diagram

The Role of Liquid Chromatography–Mass Spectrometry in the Characterization of Therapeutic Monoclonal Antibodies

IJMS | Free Full-Text | Pharmacogenomics of Anti-TNF Treatment Response Marks a New Era of Tailored Rheumatoid Arthritis Therapy
Analysis of Monoclonal Antibodies in Human Serum as a Model for Clinical Monoclonal Gammopathy by Use of 21 Tesla FT-ICR Top-Down and Middle-Down MS/MS | SpringerLink

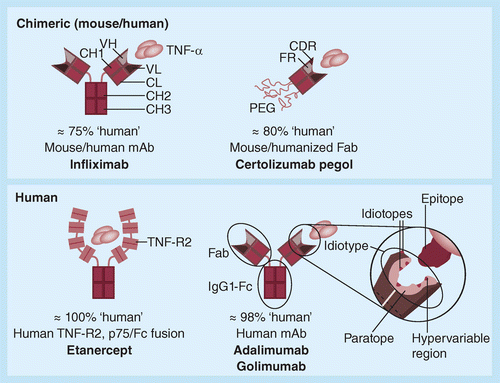





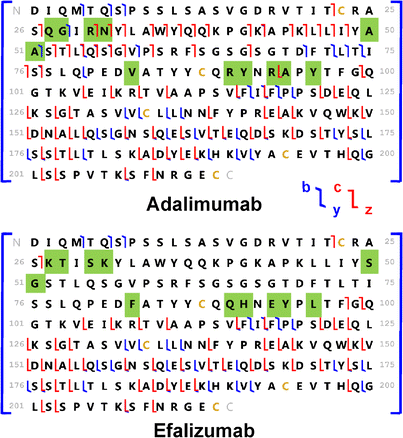
_[Adalimumab_Biosimilar_3].png)
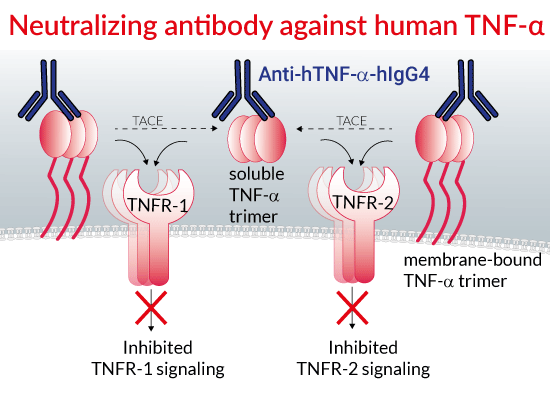

_[Adalimumab_Biosimilar_1].png)