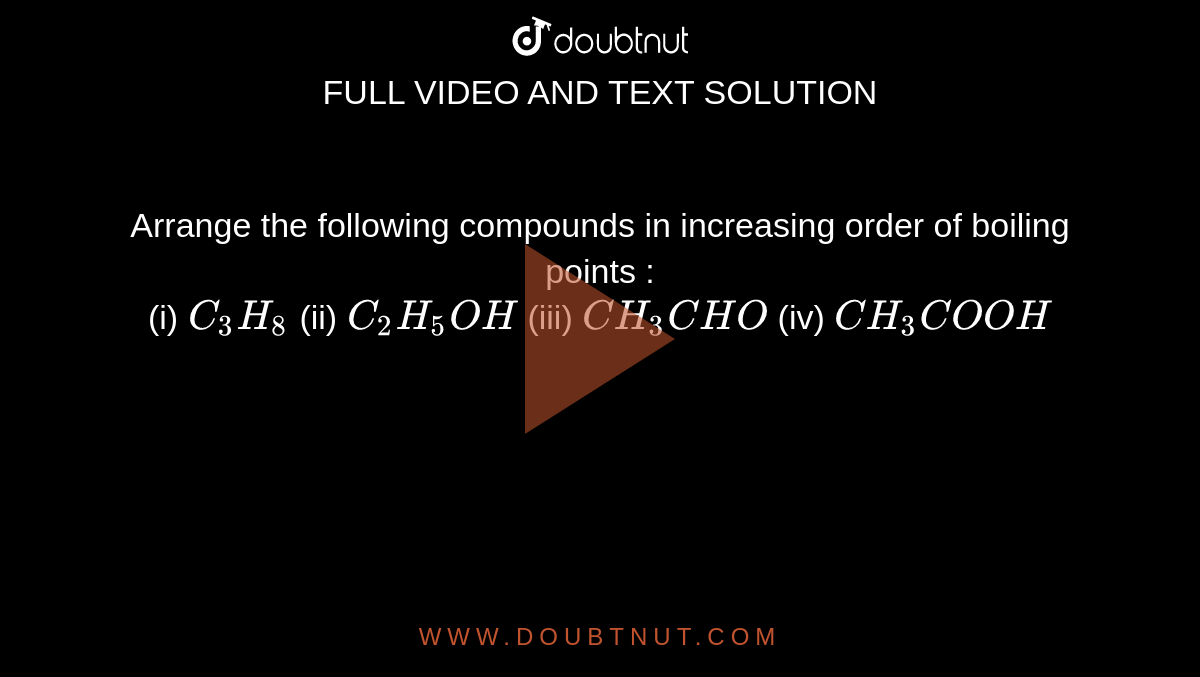
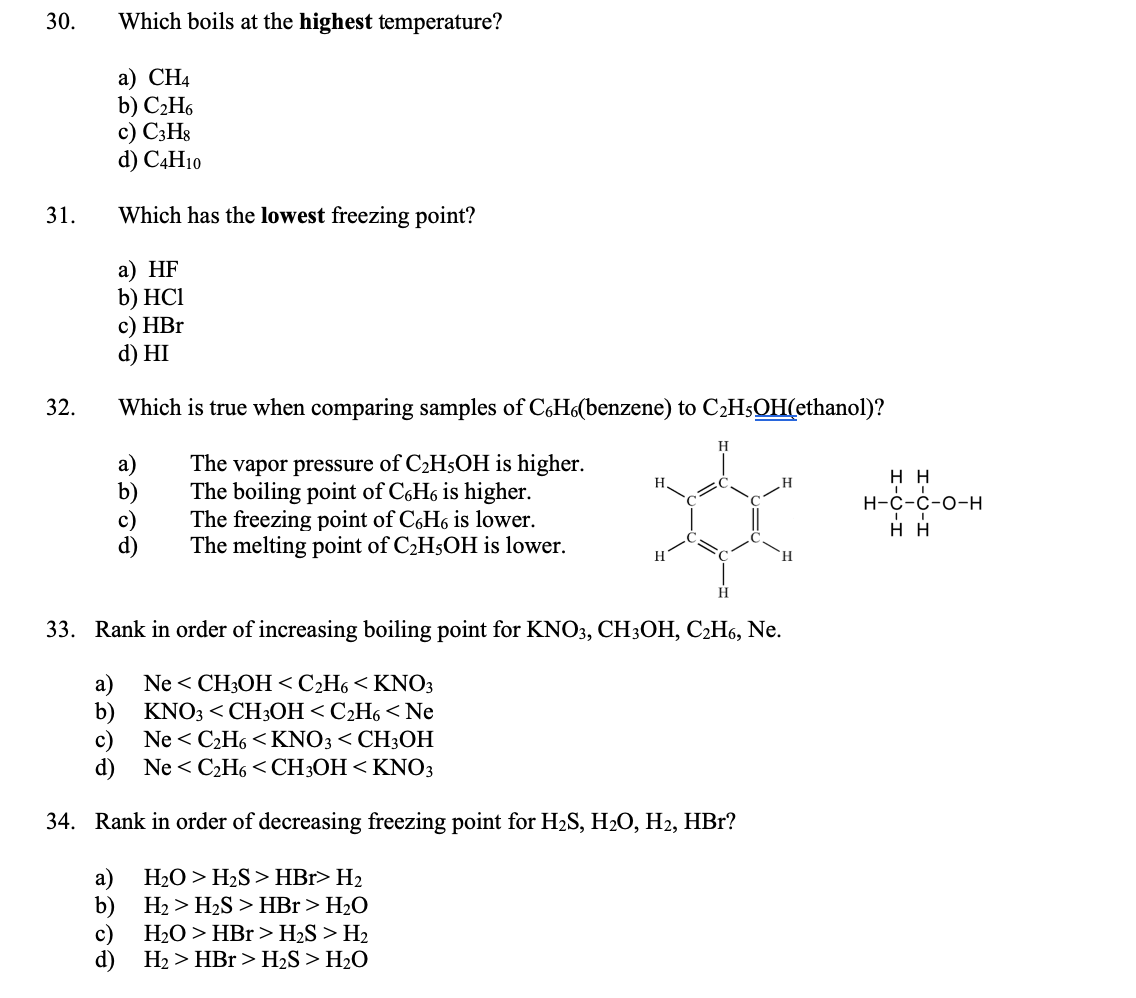
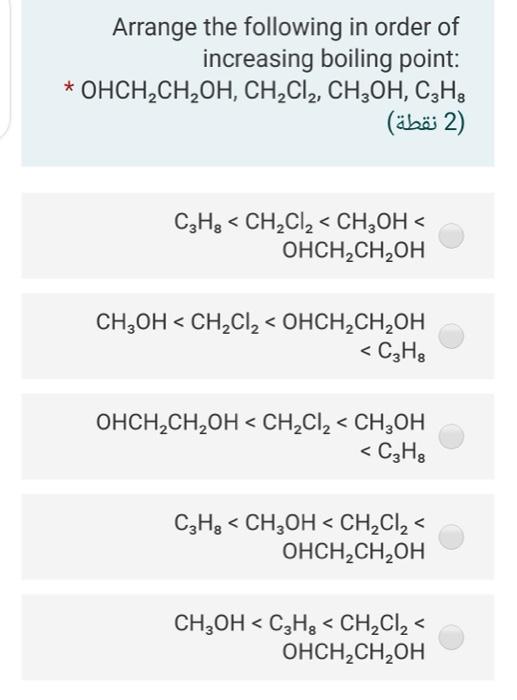
intermolecular forces - How can I determine the highest boiling point given a list of molecules? - Chemistry Stack Exchange

R290 C3h8 Hc Auto Air Conditioning Refrigerant - China Propane, Refrigernat Gas R290 | Made-in-China.com
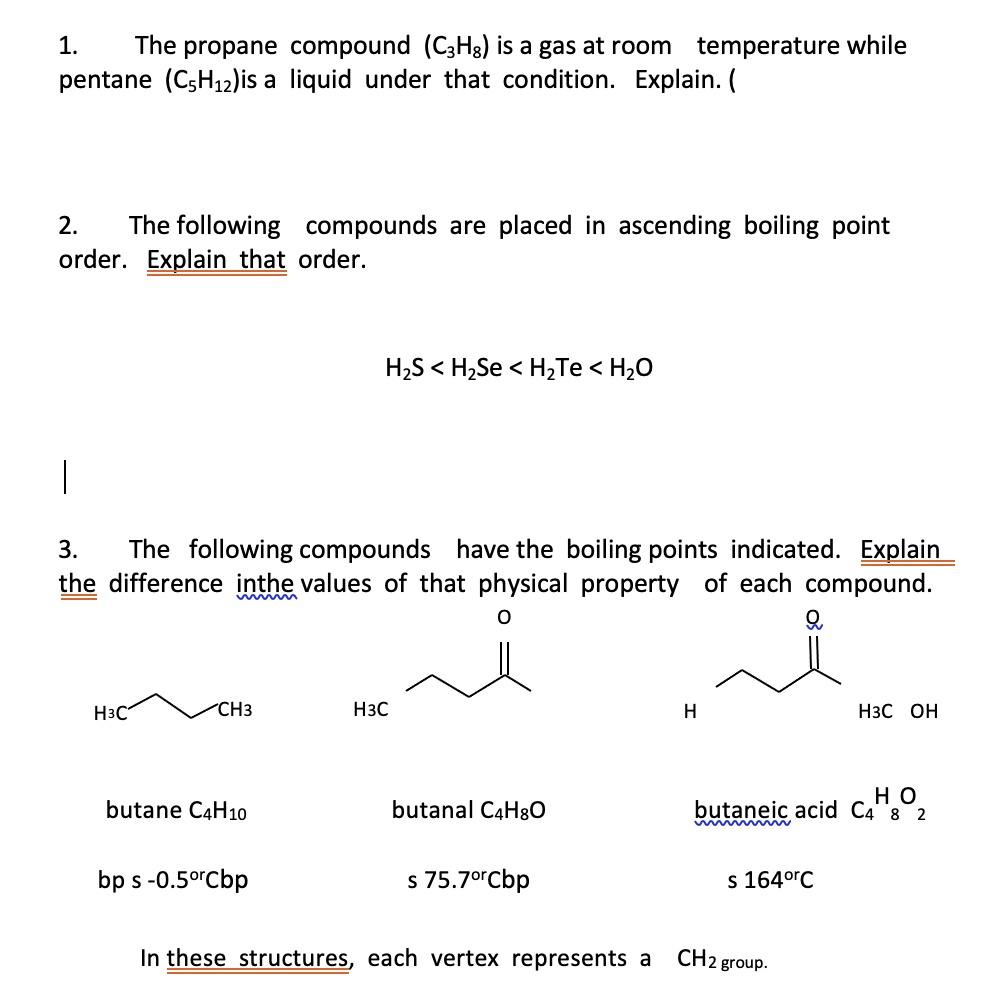
SOLVED: 1. The propane compound (CzHg) is a gas at room temperature while pentane (CsHizlis a liquid under that condition: Explain. 2 The following compounds are placed in ascending boiling point order.
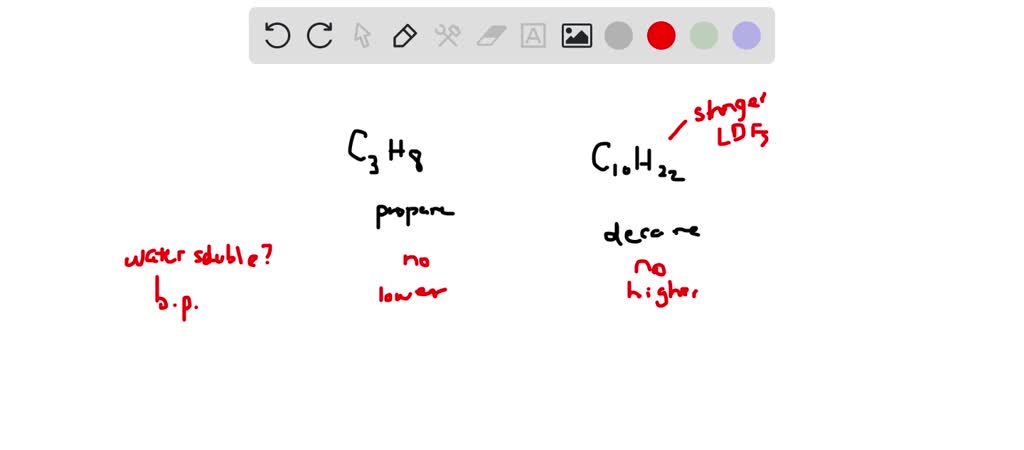
SOLVED: Consider propane (C3H8) and decane (C10H22): Which is soluble in water? Which has lower boiling point? Which has higher boiling point? Which has lower melting point? Which has higher melting point?

SOLVED: Which of the following will have the highest boiling point? A. C3H8 B. C5H12 C. C6H14 D. CH4
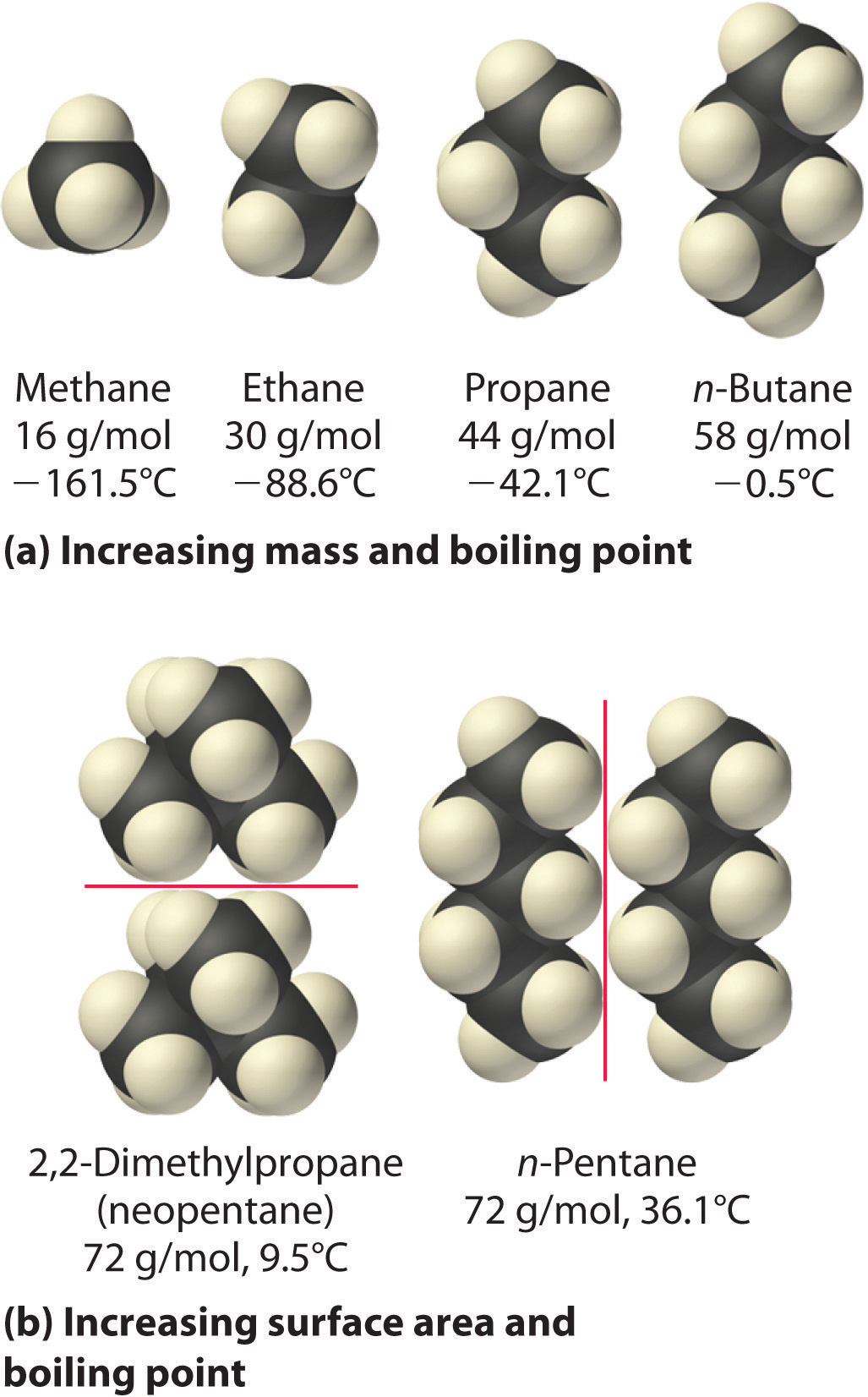
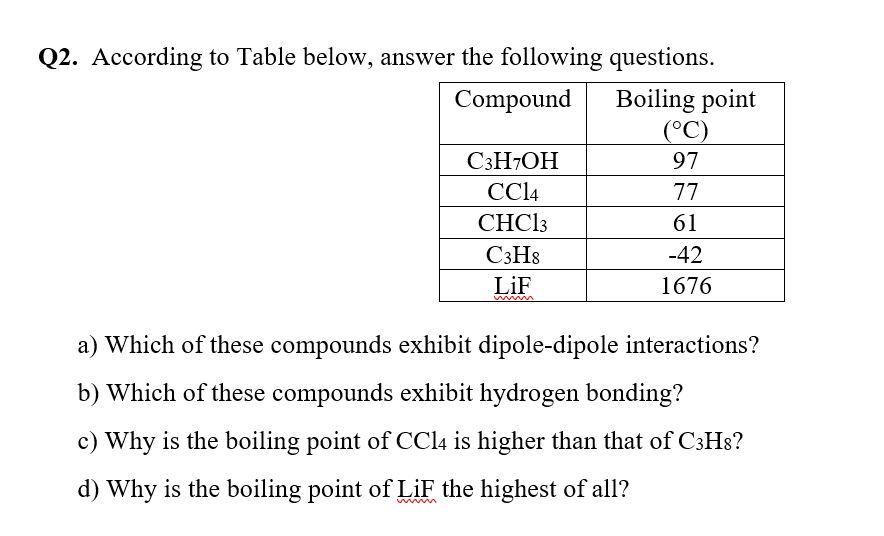


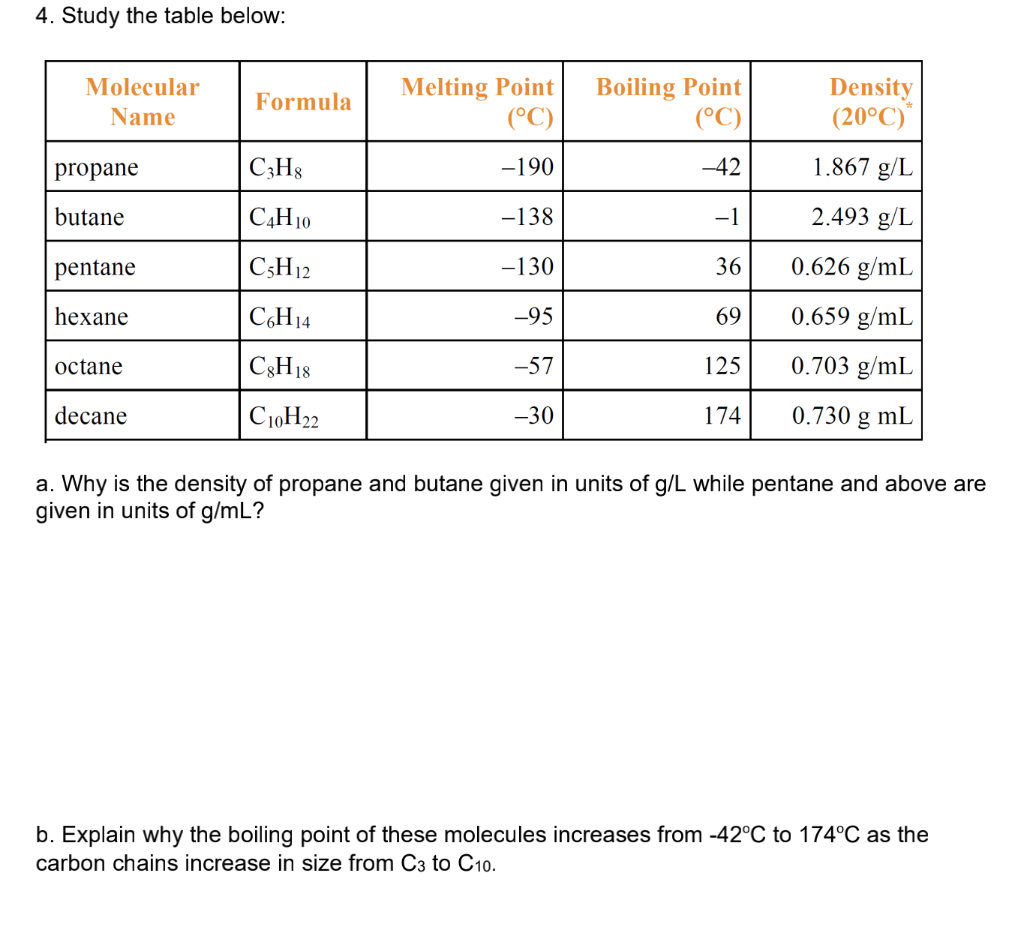
.PNG)