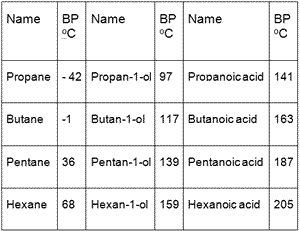
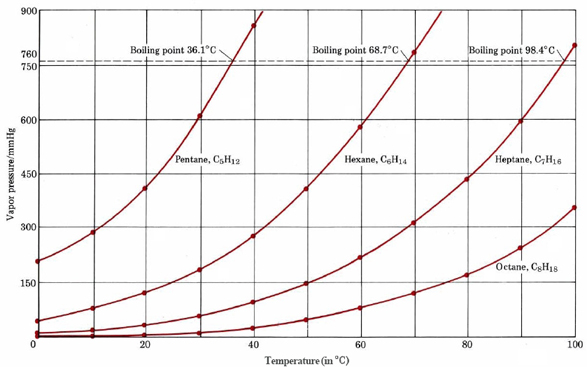
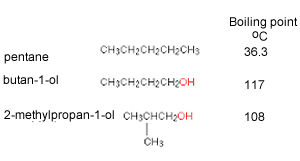
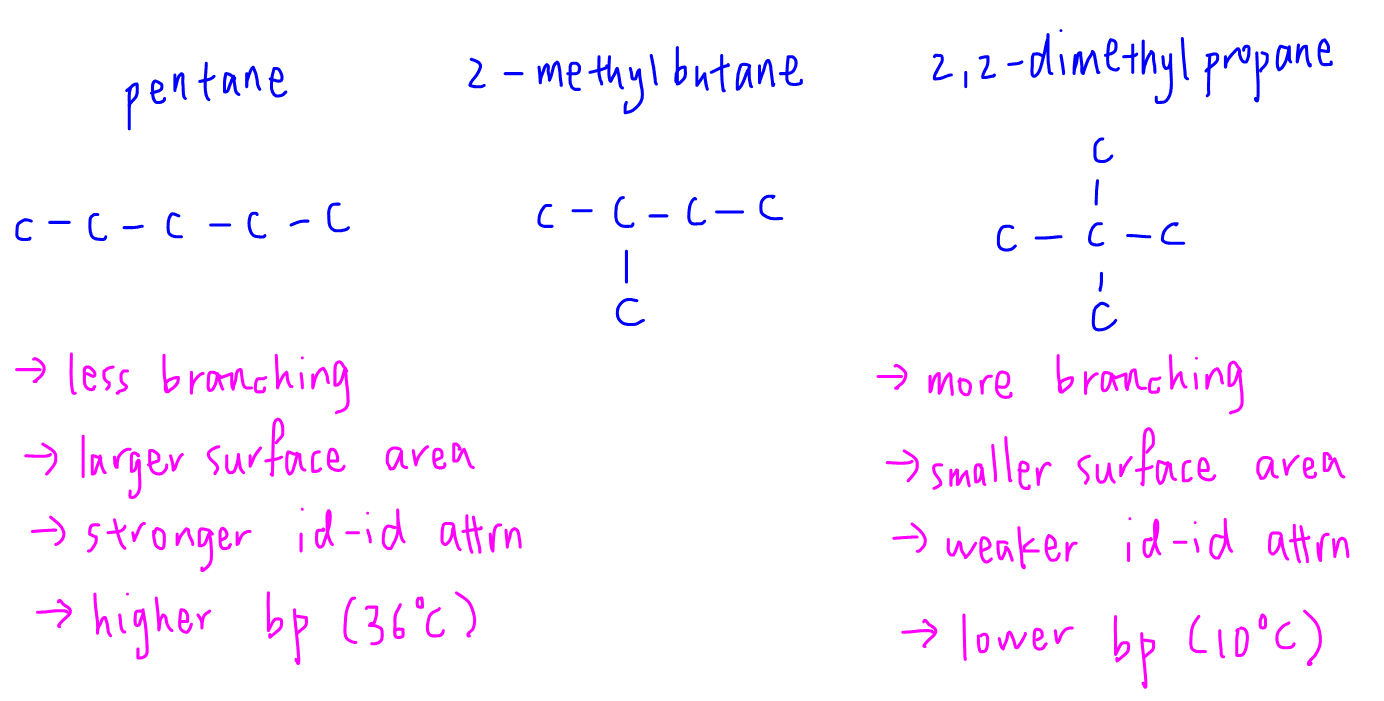Out of 2-methyl pentane and 2,3 dimethyl pentane, which has higher boiling point and why? (please give a bit - Brainly.in
Why Isomers of a compound have different Boiling point (like Isomers of pentane) why force of attraction is not involve in it? - Quora

organic chemistry - Why does neopentane have a higher melting point than n- pentane? - Chemistry Stack Exchange
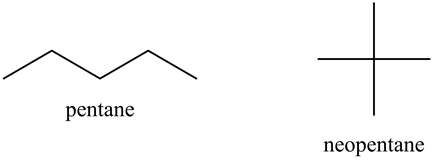
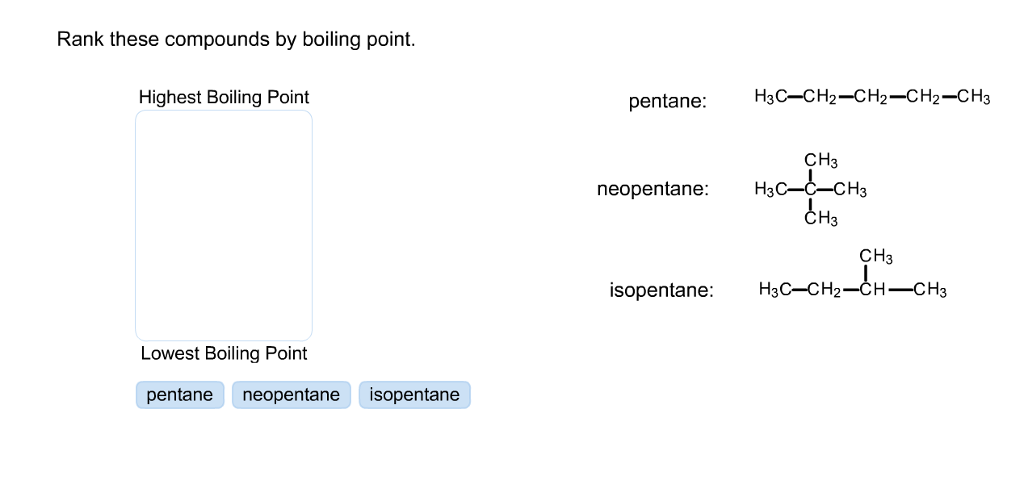
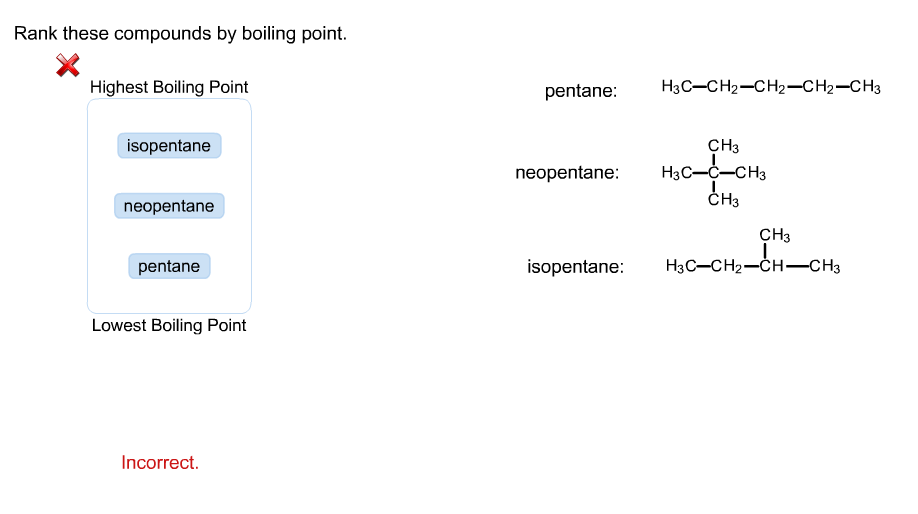
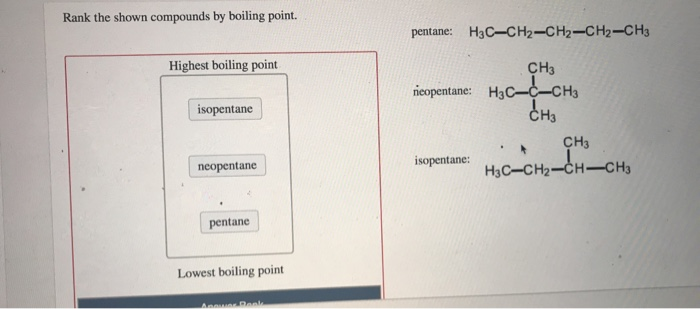
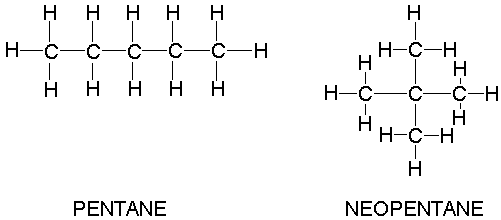
Rank these compounds by boiling point from highest to lowest boiling point: pentane, neopentane, hexane - Home Work Help - Learn CBSE Forum
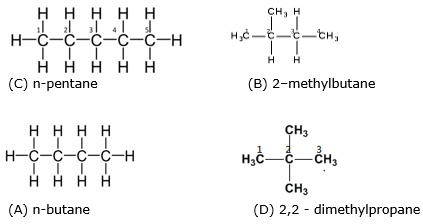
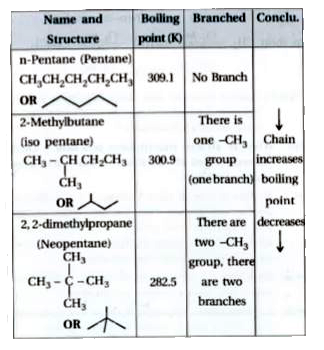
Arrange the following in decreasing order of their boiling points. (A) n–butane (B) 2–methylbutane (C) n-pentane (D) 2,2–dimethylpropane

Arrange the following compounds in the descending order of their boiling pointsa) n - pentaneb) isopentanec) neopentane

Arrange the following compounds in the descending order of their boiling pointsa) n - pentaneb) isopentanec) neopentane
21. Why The boiling point of pentane is greater than isopentane? And why the boiling point of neopentane is less than N pentane and isopentane?
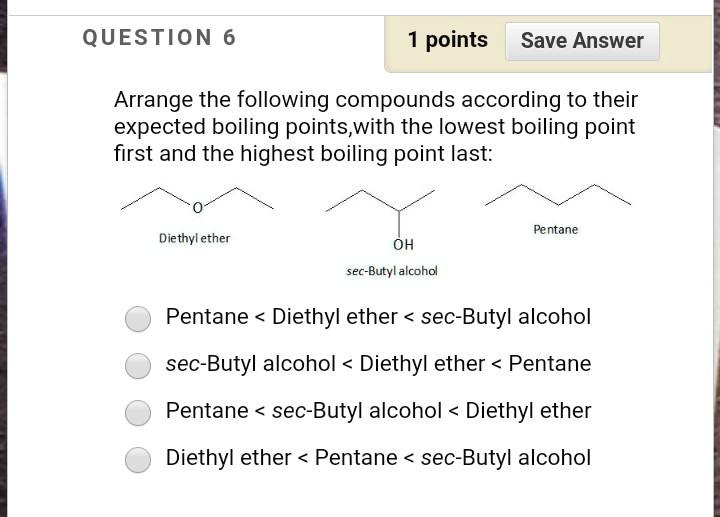
SOLVED: QUESTION points Save Answer Arrange the following compounds according to their expected boiling points,with the lowest boiling point first and the highest boiling point last: Pentane Diethyl ether OH sec-Butyl Icohol
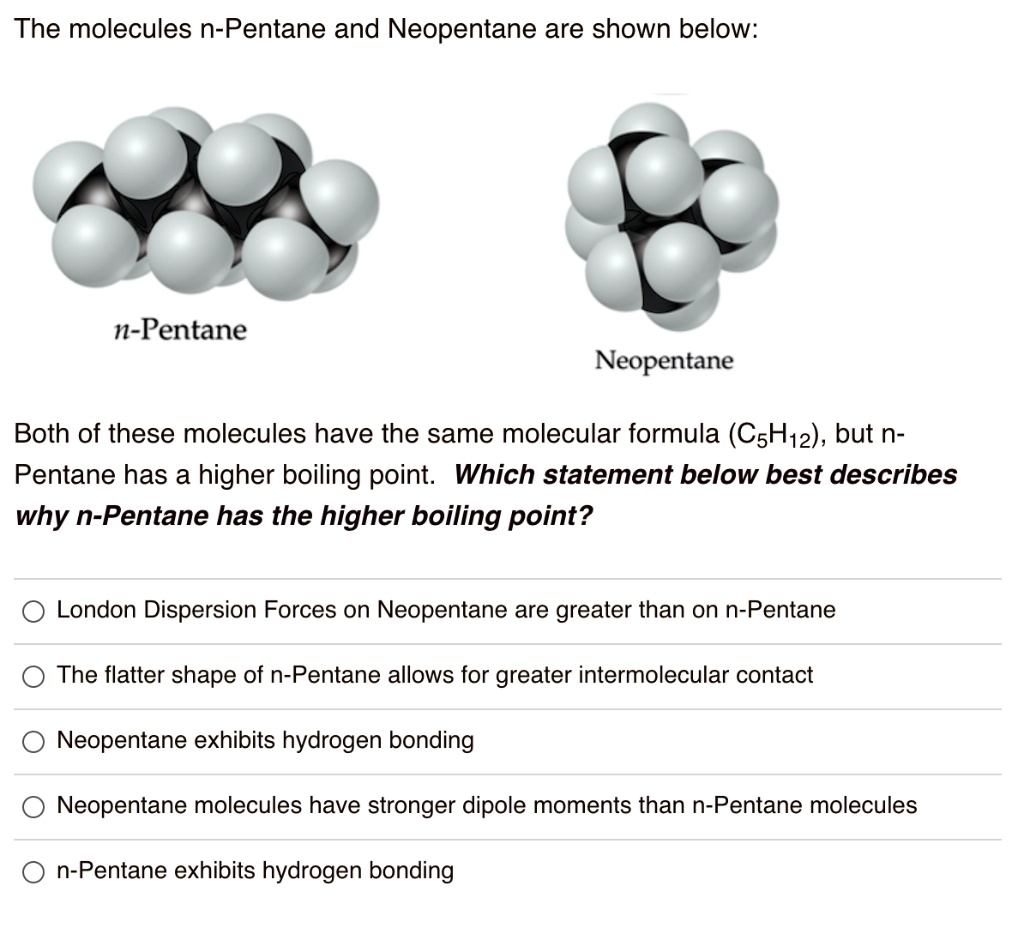
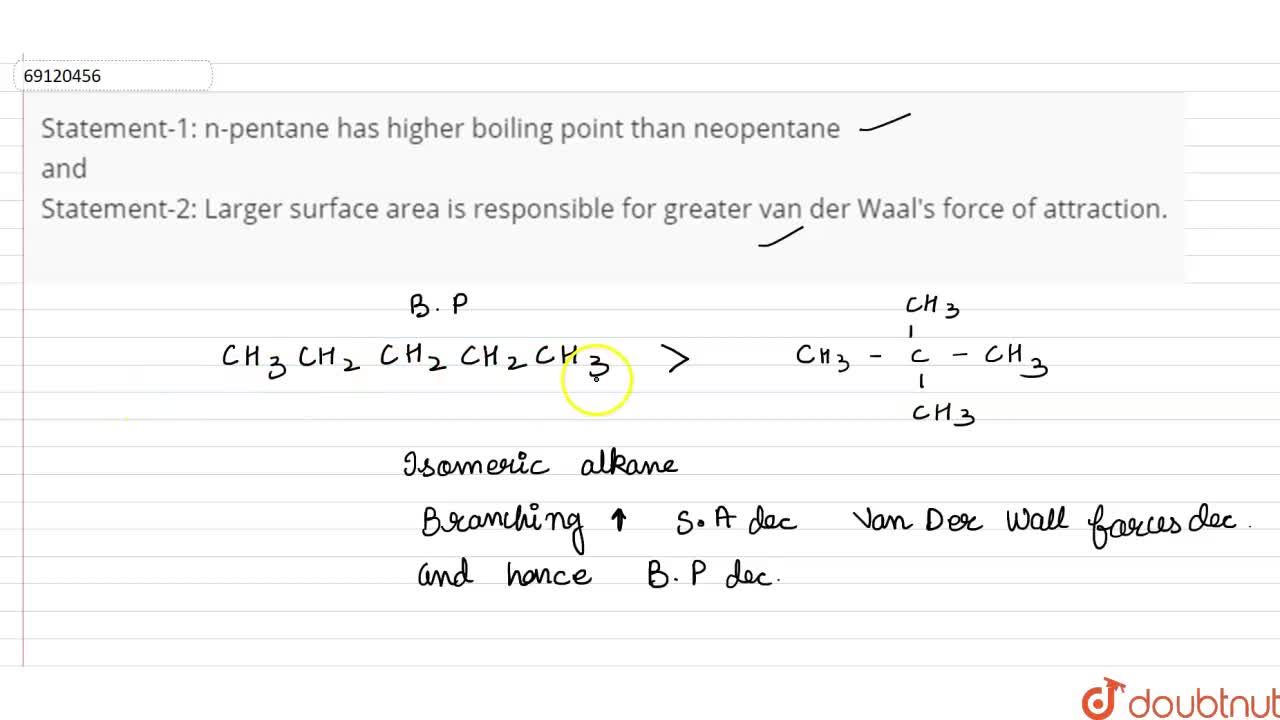
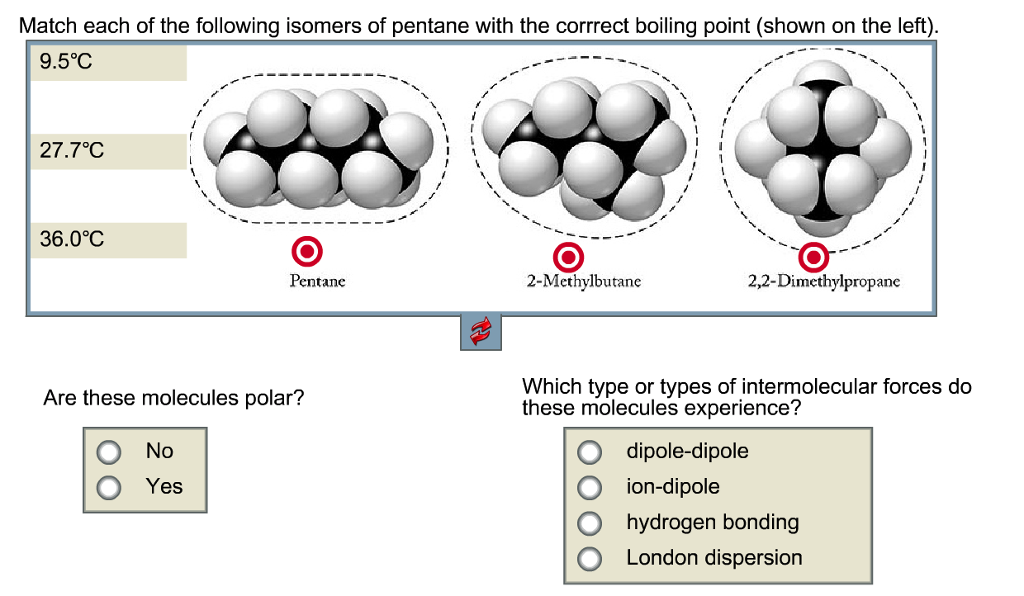
SOLVED: The molecules n-Pentane and Neopentane are shown below: n-Pentane Neopentane Both of these molecules have the same molecular formula (C5H12), but n- Pentane has a higher boiling point: Which statement below
Why when the shape of molecules become more compact it's boiling point decrease while when intermolecular force become strong boiling point increase? | Socratic