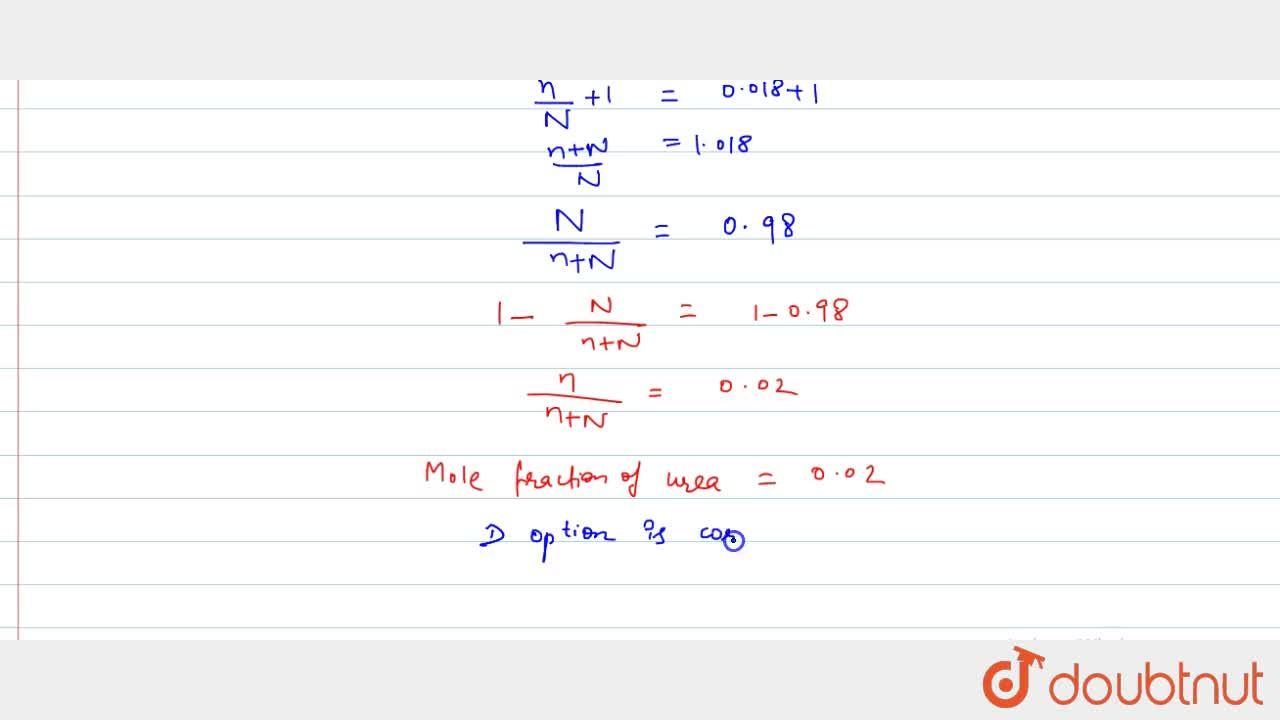
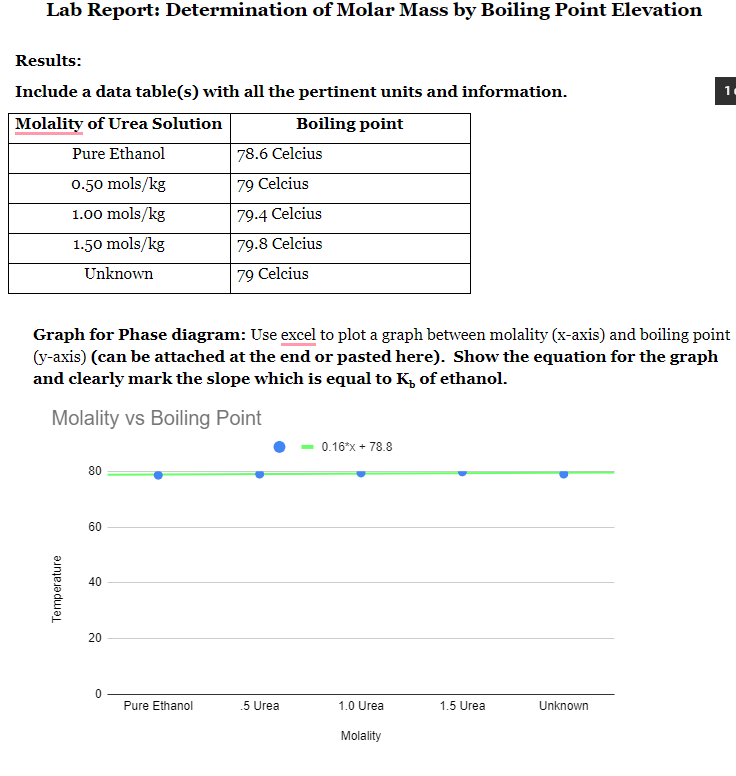
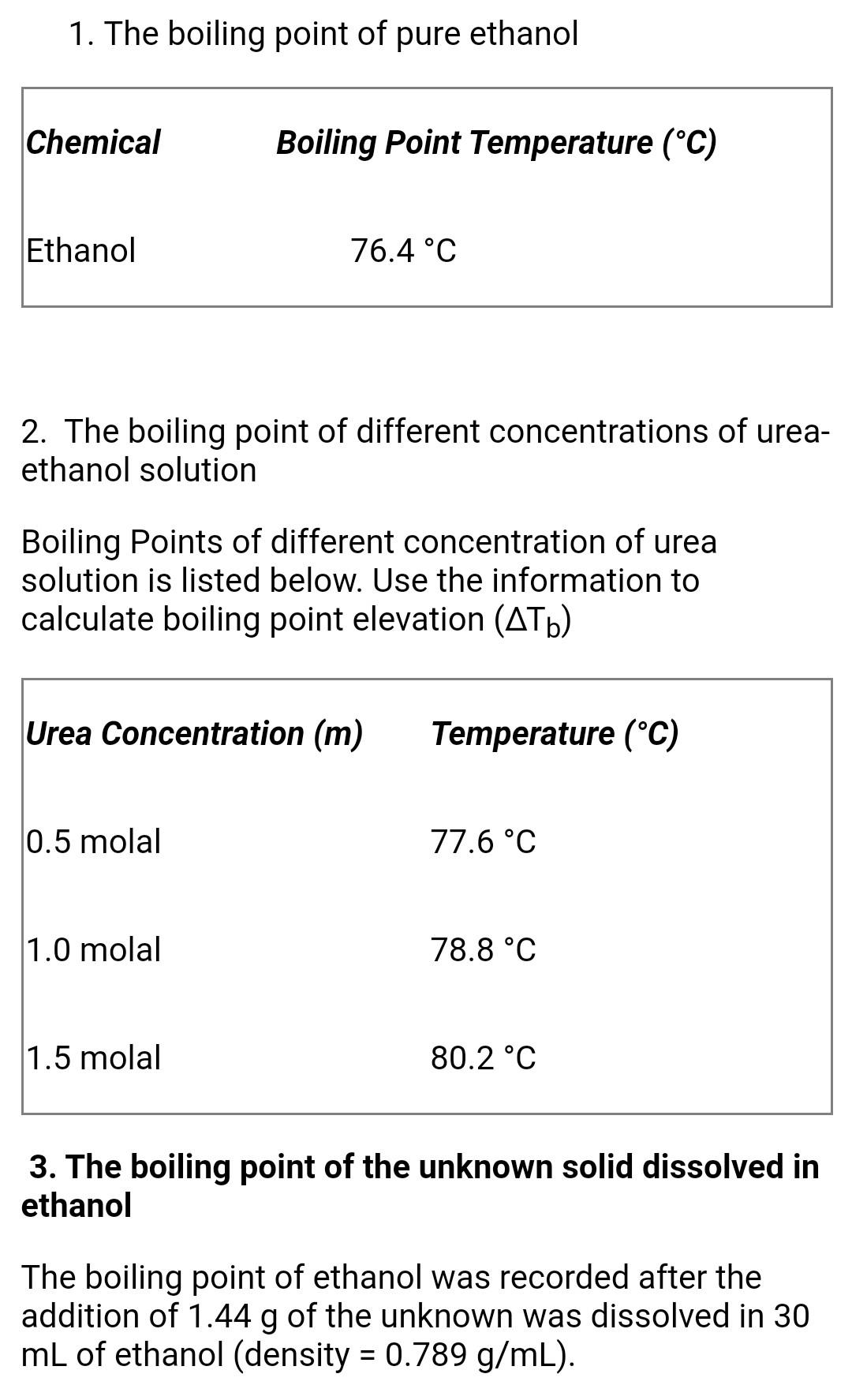
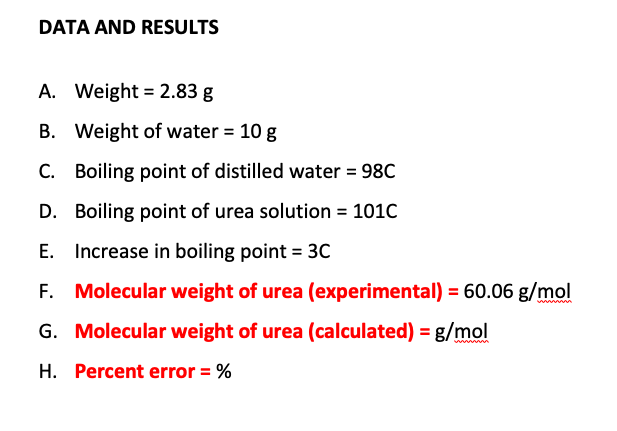
SOLVED: certain substance X has normal boiling point of 127.4 -C and mola boiling point elevation constant Kb= 1.04 *€*kg mol dissolving some urea ((NHz),c0) in 300. g of X. This solution
Elevation in boiling point of an aqueous urea solution is 0.52° (Kb = 0.52° mol^-1 kg). - Sarthaks eConnect | Largest Online Education Community

Solve this: 49 Which have highest boiling point 1% urea solution (2) 1% glucose (3) 1% sucrose - Chemistry - Solutions - 11642483 | Meritnation.com

Determine the boiling points of 1 m solution of sugar, glucose, urea, sodium chloride, barium chloride, aluminium chloride. | Homework.Study.com
Calculate the boiling point of urea solution when 6 g of urea is dissolved in 200 g of water. (Kb for water = 0.52 K kg mol^-1 , - Sarthaks eConnect | Largest Online Education Community

The molal elevation constant of water is 0.51 . The boiling point of 0.1 molal aqueous NaCl solution is nearly:

What will be boiling point of 0.1 m Urea(aq) solution?( kb = 0.52 K Kg mo1 -1 )(A) 273.67 K(B) 373.2 K (C) - Brainly.in

Statement The boiling point of `0.1 M` urea solution is less than that if `0.1M KCl` solution. - YouTube
Aqueous solutions in the order of their increasing boiling points 1) 0.0001 M NaCl 2)0.001 M MgCl2 3)0.001 M urea 4) 0.001 M Nacl

A solution of urea in water has boiling point of `100.15^()C`. Calculate the freezing point of t... - YouTube
14. A urea solution has boiling point 373.128K then what is its melting point ? Given kf =1.86 and kb =0.52.

The boiling points for aqueous solutions of sucrose and urea are the same at a constant temperature. If 3gm of urea is dissolved in its 1 litre solution, what is the weight

What will be boiling point of 0 1 m Urea(aq) solution ( kb = 0 52 K Kg mol-1) - Chemistry - Solutions - 15085683 | Meritnation.com

87564-10-1 Urea - formaldehyde ammoniate (1:1:1) C2H9N3O2 NMR,Molecular Structure, Molecular Formula,Boiling Point, Flash Point, -Wörterbuch - guidechem.com