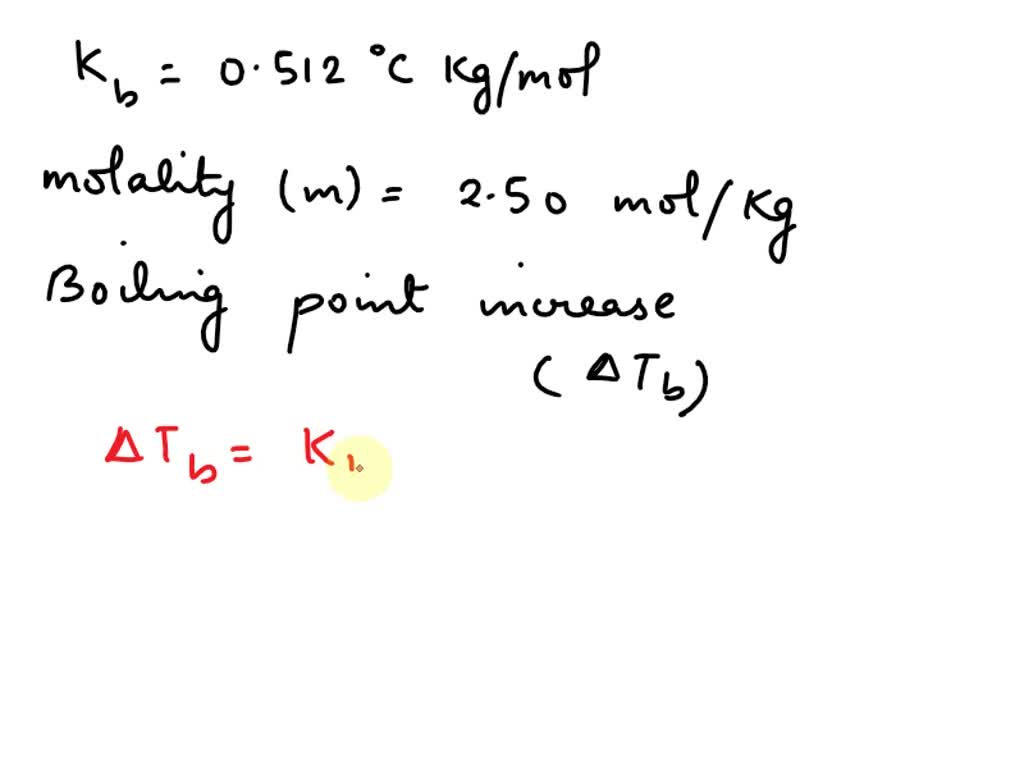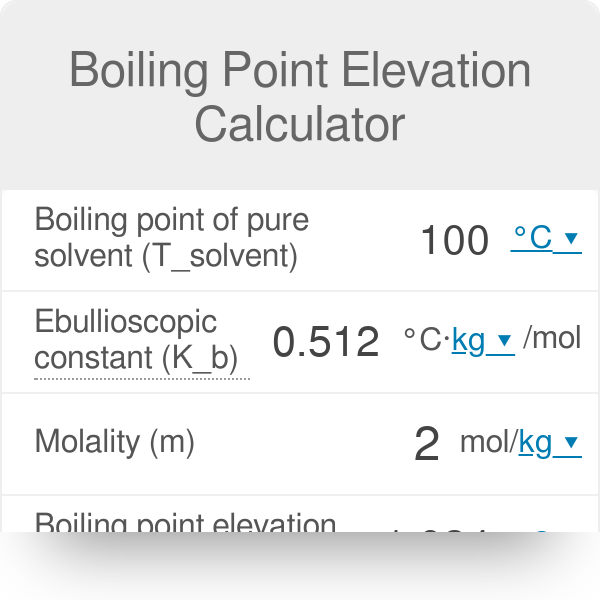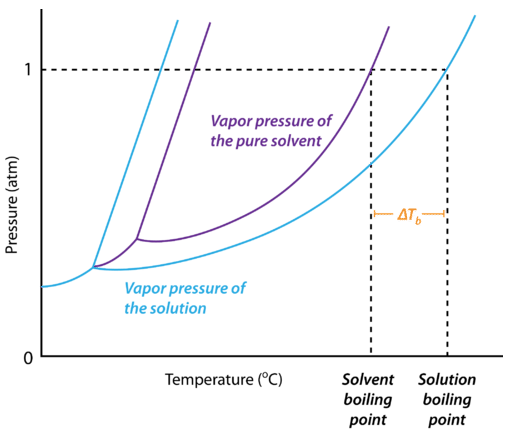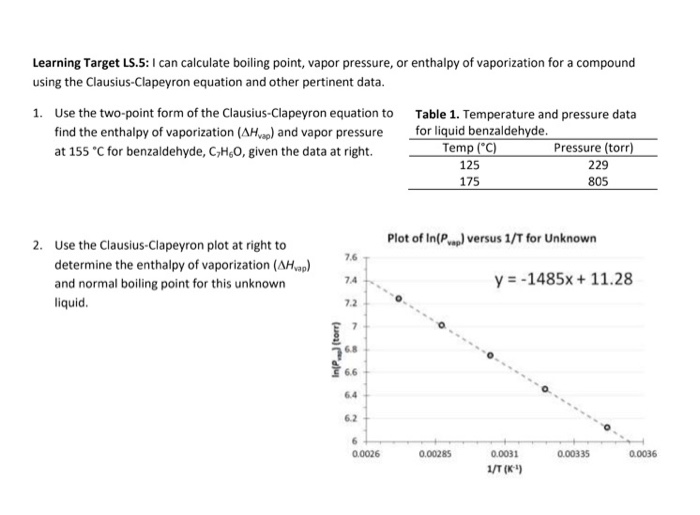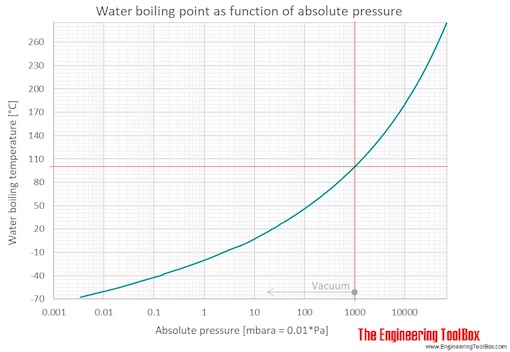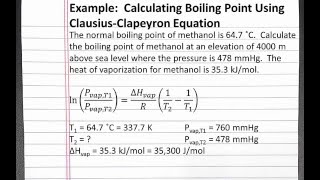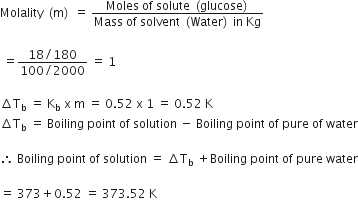
The boiling point of pure water is 373K. Calculate the boiling point of an aqueous solution containing 18 gms of glucose (MW = 180) in 100 gms of water. Molal elevation constant

Boiling Point Elevation and Freezing Point depression - Example 2 ( Video ) | Chemistry | CK-12 Foundation
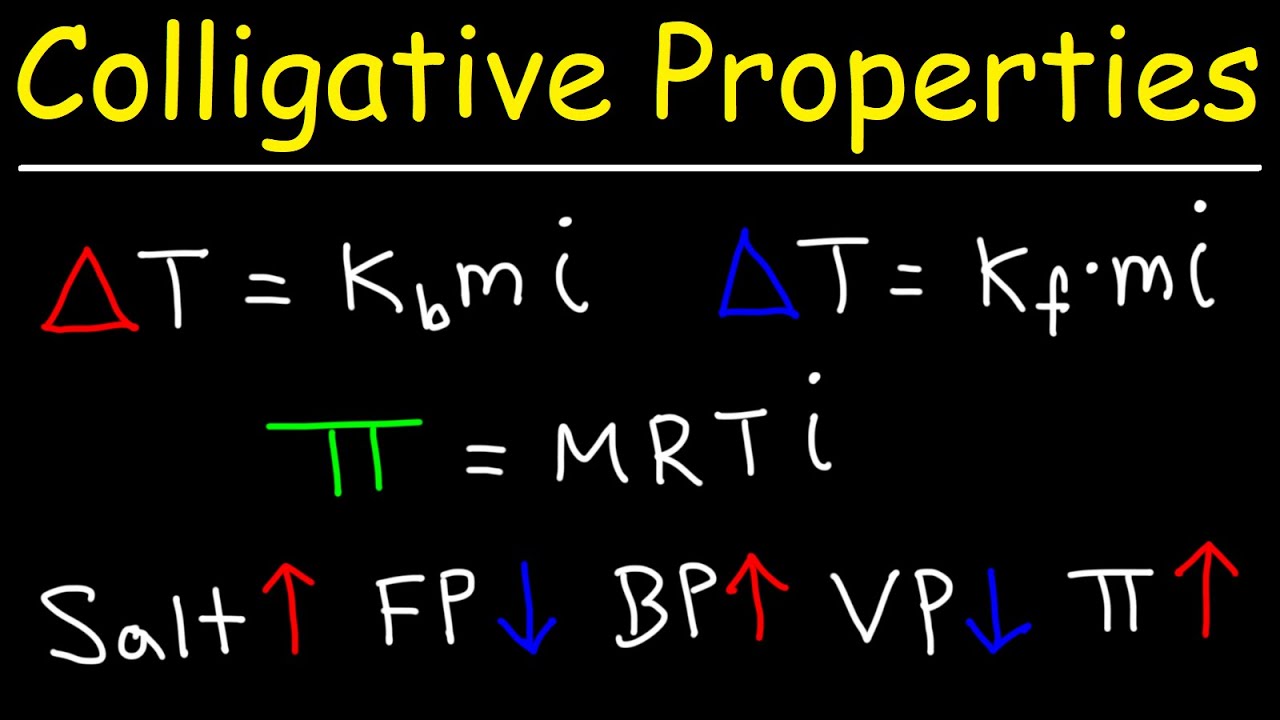
Colligative Properties - Boiling Point Elevation, Freezing Point Depression & Osmotic Pressure - YouTube

Calculate the freezing point and the boiling point at 1 atmosphere of a solution containing 30 g cane sugar (molecular mass 342 ) and 150 g water.Given : Kb = 0.513 and Kf = 1.86

OneClass: Calculate the normal boiling point of a compound that has a vaporpressure of 500 torr at 20...

Determine the normal boiling point in K of a substance whose vapor pressure is 55.1 mmHg at 23.2^o C and has a ?H_vap of 32.1 kJ/mol. | Homework.Study.com
Calculate the boiling point of solution when 4 g of MgSO4 (M = 120 g mol^-1) was dissolved in 100 g of water assuming - Sarthaks eConnect | Largest Online Education Community