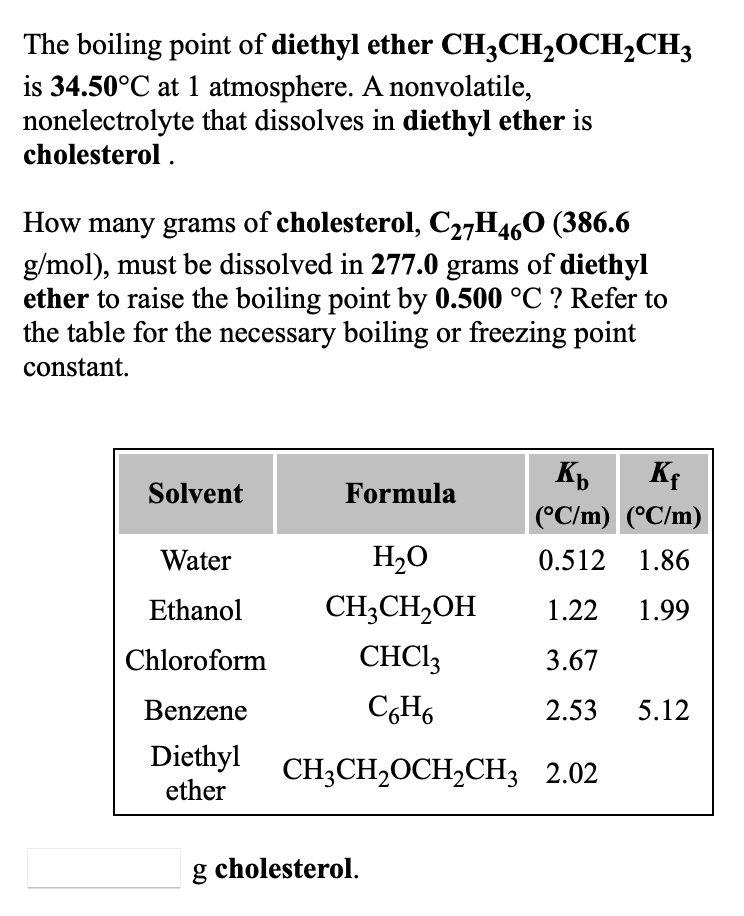
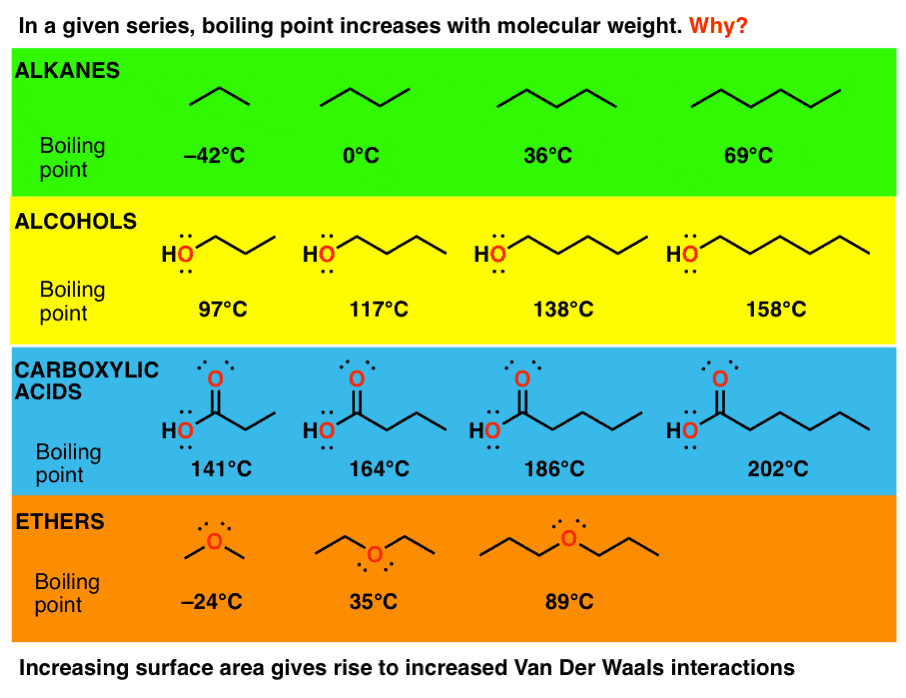
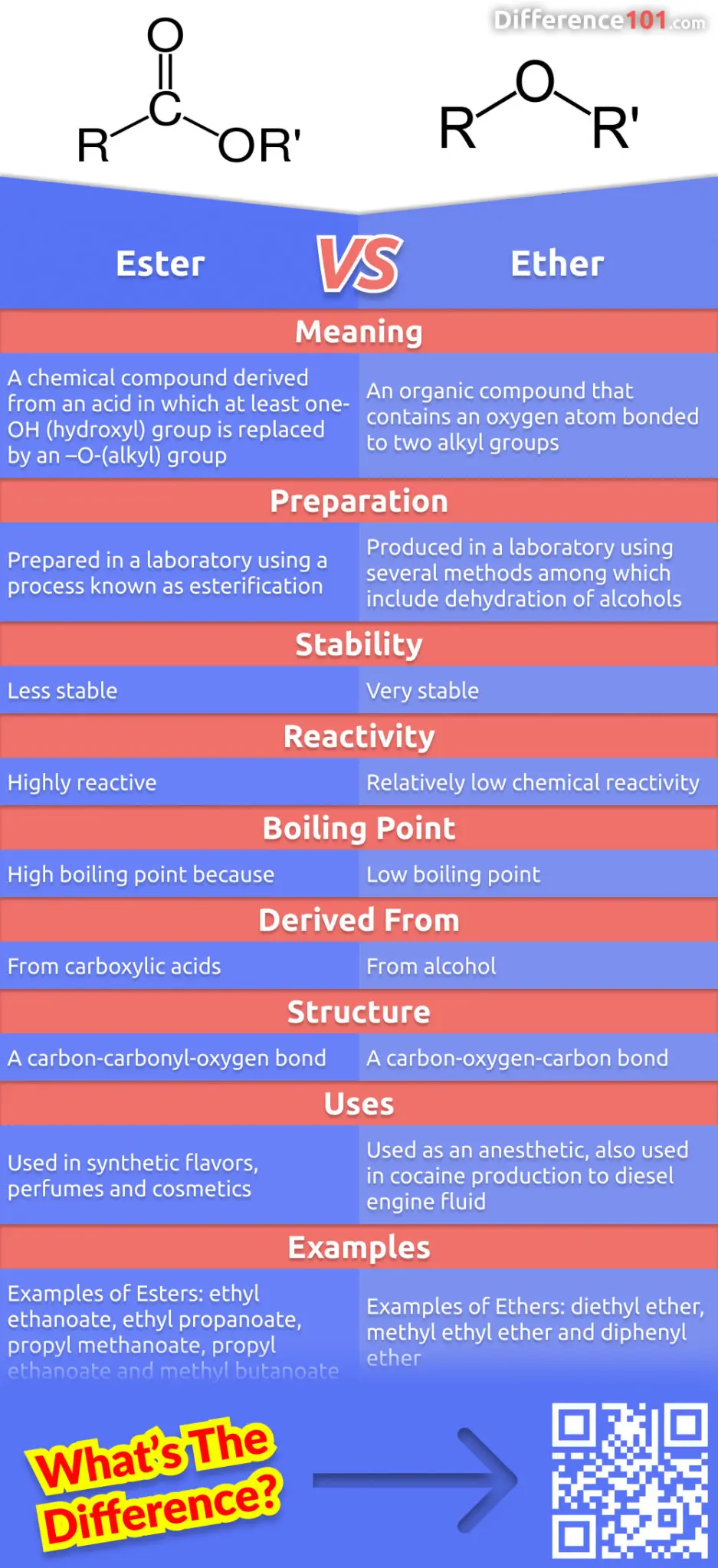
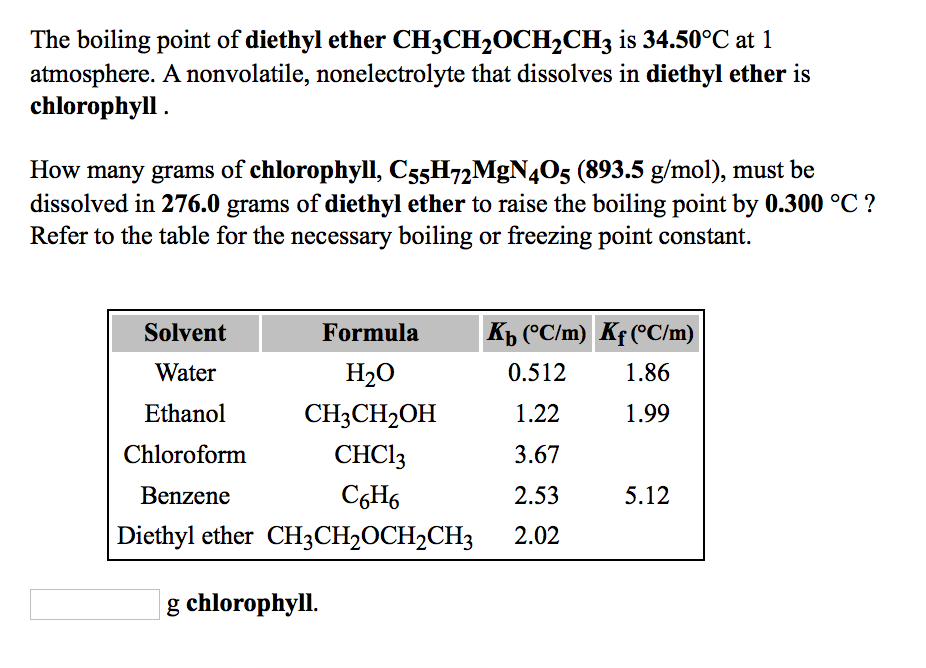
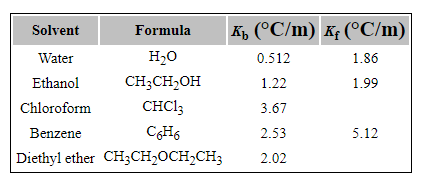
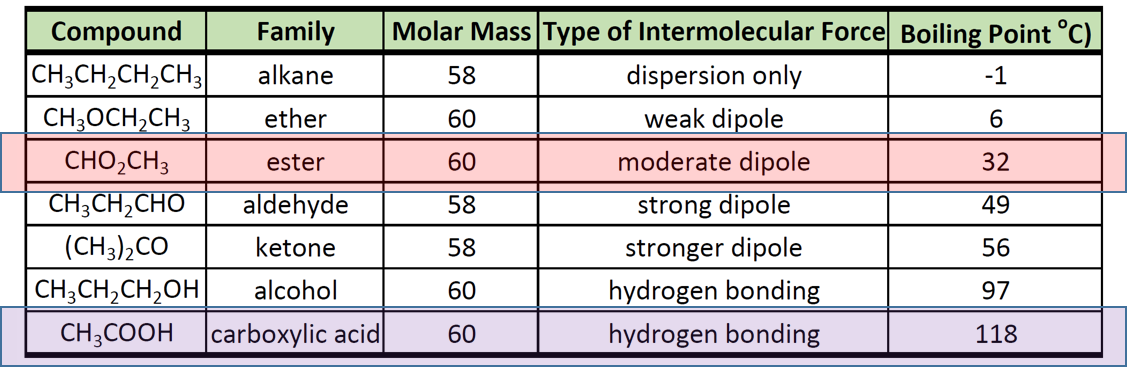
In general, ethers have a much lower boiling point than their isomeric alcohols. Why? A. The carbon-oxygen bond in ethers is nonpolar. B. Unlike alcohols, ethers cannot act as Lewis bases. C.

Properties of Alcohols, Ethers, and Thiols Chapter 12 Organic Compounds with Oxygen and Sulfur Copyright © 2005 by Pearson Education, Inc. Publishing. - ppt download
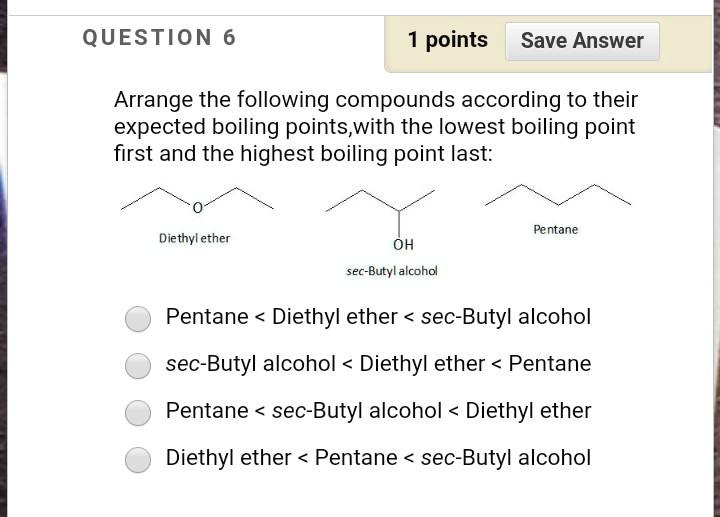
SOLVED: QUESTION points Save Answer Arrange the following compounds according to their expected boiling points,with the lowest boiling point first and the highest boiling point last: Pentane Diethyl ether OH sec-Butyl Icohol
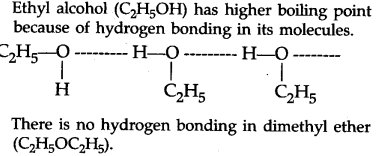
Which one has high boiling point ethyl alcohol or dimethyl ether and why? - CBSE Class 11 Chemistry - Learn CBSE Forum

organic chemistry - Why do alcohols and ethers have approximately the same solubility in water but different boiling points? - Chemistry Stack Exchange

Diethyl ether has a much higher boiling point than butane despite having a higher molecular weight. Explain why this is the case, making reference to the molecular structures of both compounds.