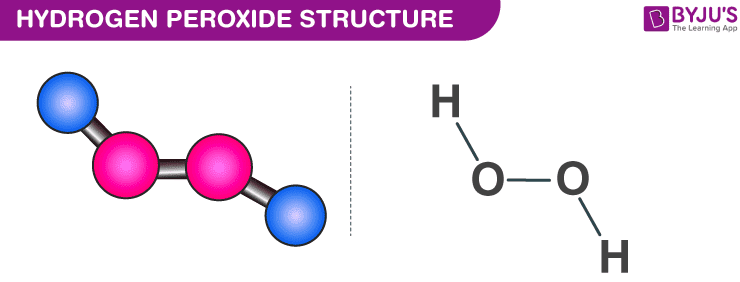
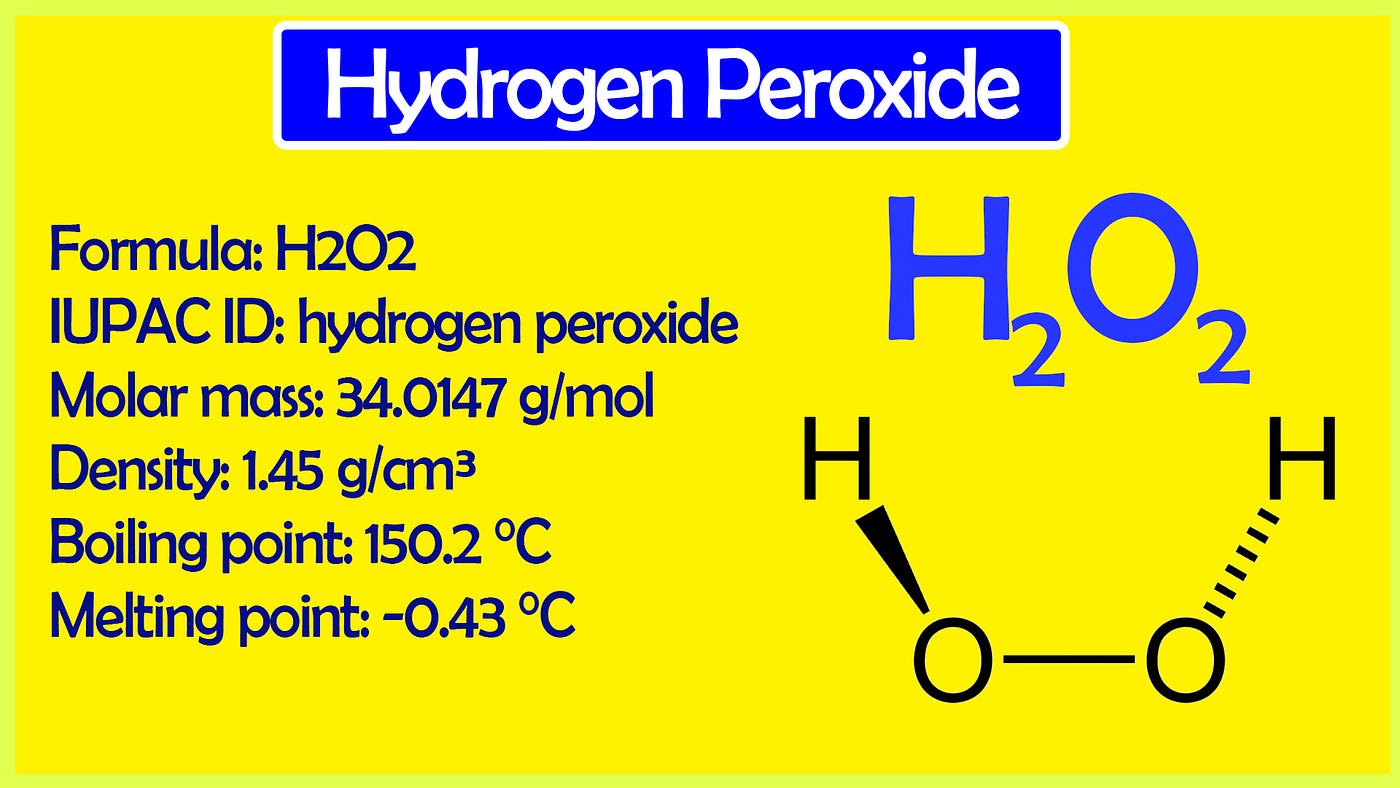
Which of the following is not a proper match(A) D-D is equal to H-H........ Bond length(B) H20 Ꭰ20 , H2O2 - Brainly.in

Effect of temperature and H2O2 concentration on the carboxyl groups'... | Download Scientific Diagram
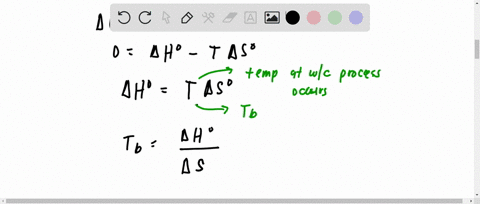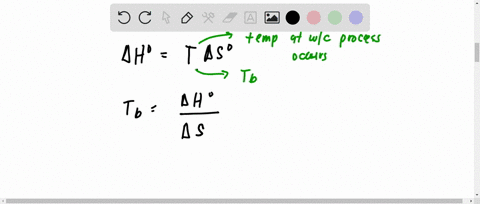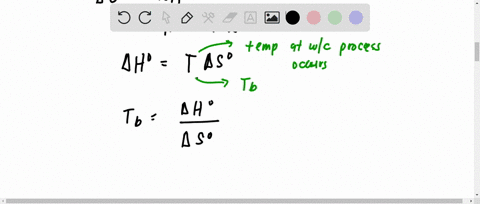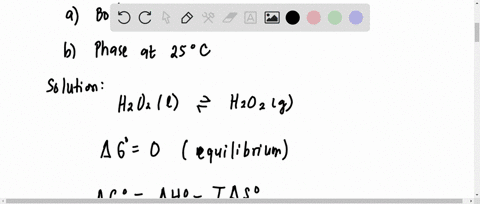
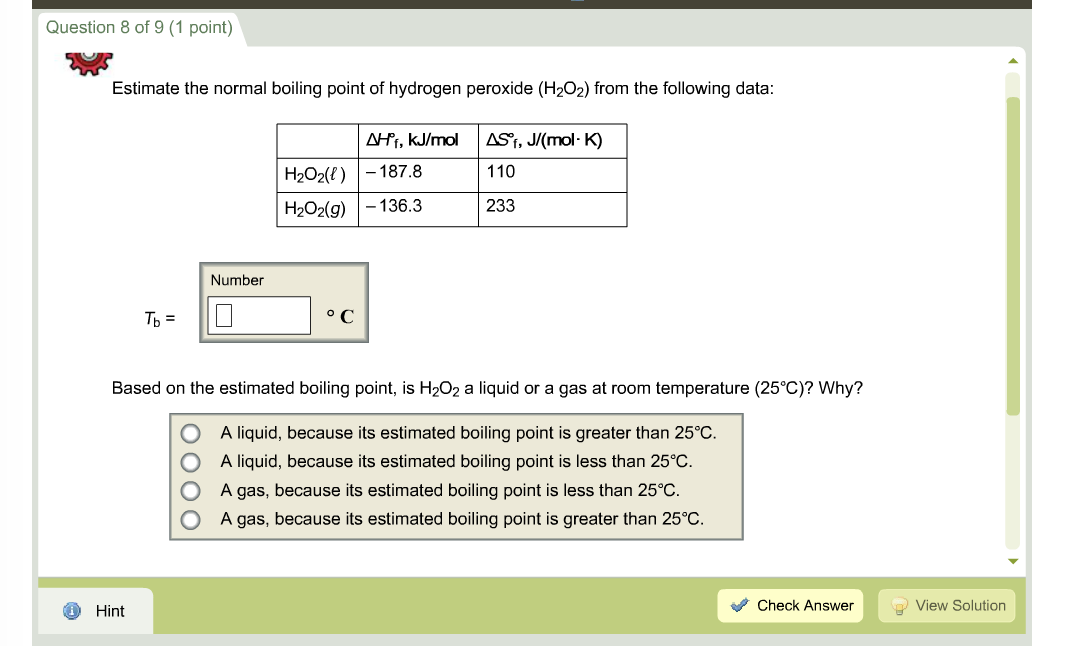
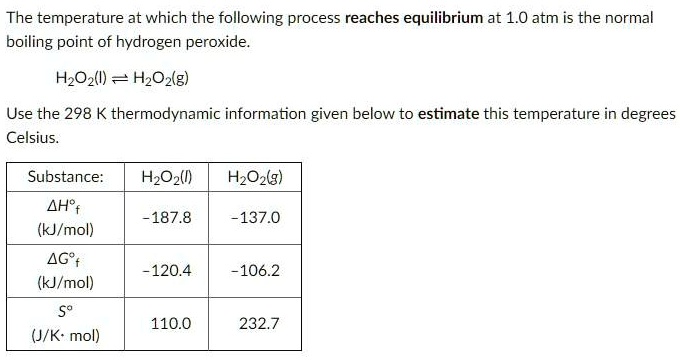
SOLVED:Hydrogen peroxide, H2 O2, has a normal boiling point of 150^∘ C. Based on the data given in Figure 11.25 , would you expect hydrogen peroxide to have a higher or lower
Sciencemadness Discussion Board - Hydrogen Peroxide - Illustrated Practical Guide - Powered by XMB 1.9.11

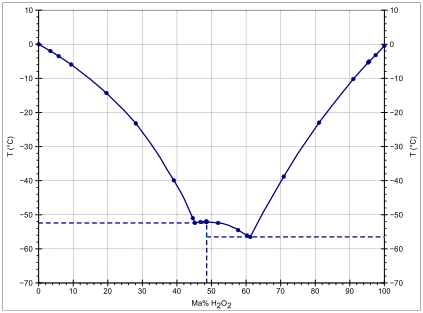

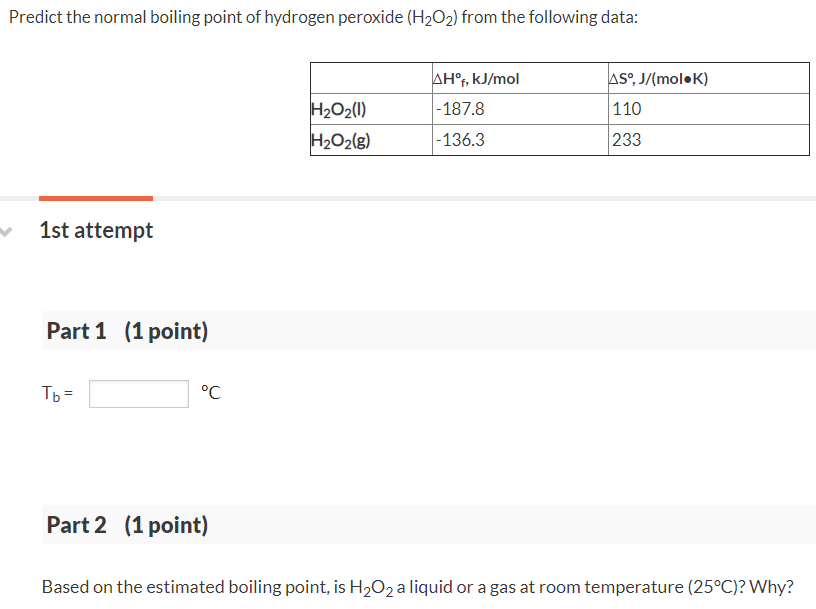






.gif)