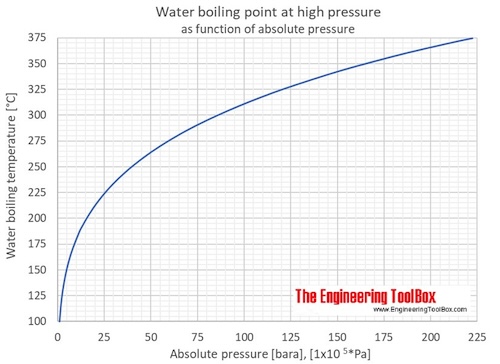
Predicted boiling point temperature values in unit of K for selected... | Download Scientific Diagram
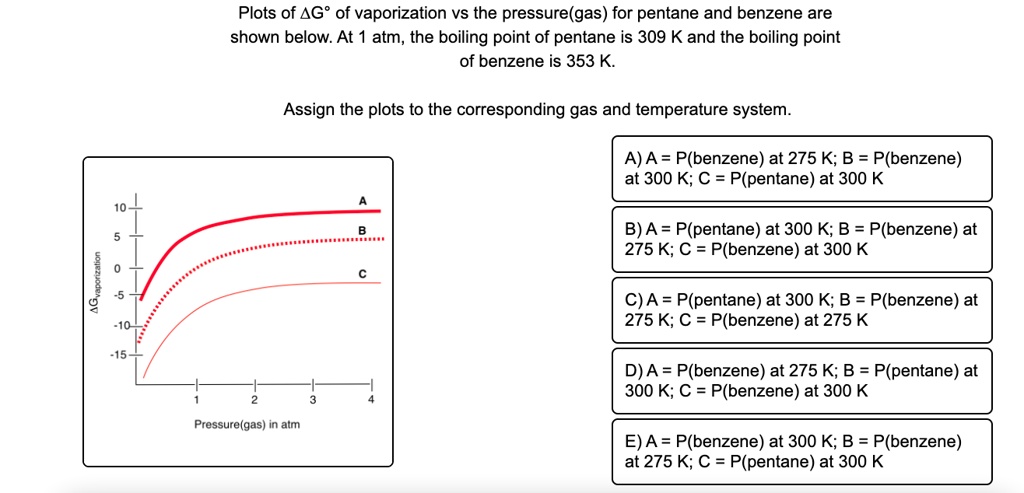
SOLVED: Plots of AG? of vaporization vs the pressure(gas) for pentane and benzene are shown below: At atm, the boiling point of pentane is 309 K and the boiling point of benzene

The normal boiling point of water is 373 k. vapour of waterr at temperature T is 19 mm hg. If en... - YouTube
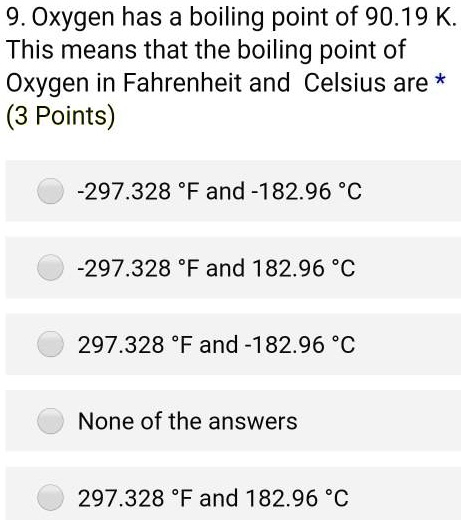
SOLVED: 9. Oxygen has a boiling point of 90.19 K This means that the boiling point of Oxygen in Fahrenheit and Celsius are * (3 Points) 2297.328 F and-182.96 %C 297.328 Fand

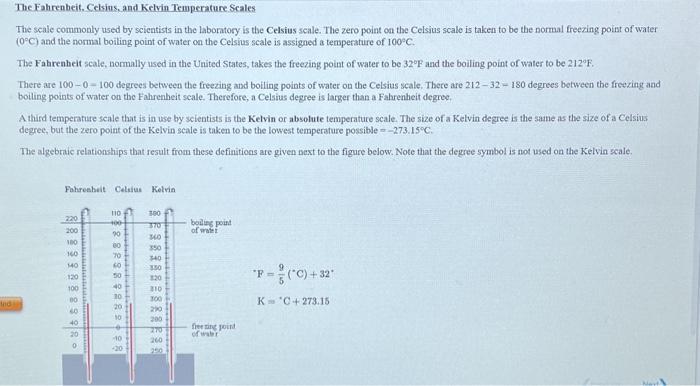
Temperature Temperature Scales Fahrenheit 212 o F 180 o F 32 o F Celcius 100 o C 0 o C Kelvin 373 K 100 K 273 K Boiling point of water Freezing point. - ppt download