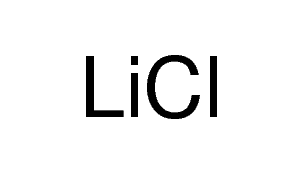SOLVED: Place the following compounds in order of increasing boiling point: LiCl F2 CHBCHzNHz A LiCl < CH3CHzNHz < Fz B. F2 LiCl CH3CHzNH 2 C.F2 CH3CHzNH2 < LiCl D: CH3CHzNHz LiCl <

Licl Lithium Chloride Anhydrous CAS 7447-41-8 with Customized Packing - China Lithium Chloride, Licl | Made-in-China.com

Solubility measurement and prediction of phase equilibria in the quaternary system LiCl + NaCl + KCl + H2O and ternary subsystem LiCl + NaCl + H2O at 288.15 K - ScienceDirect

SOLVED: LiCl has higher boiling point then Hcl explain on the basis of binding bsc 1st year chemistry

CHEM1011 - Solution A Hcl Would Have A Lower Boiling Point Than Licl Because The Dipole | Course Hero

Pressure-temperature relations of lithium chloride ammonia complexes... | Download Scientific Diagram

Competitive Price Licl Monohydrate Cas No 7447-41-8 Lithium Chloride - Buy Lithium Chloride,Licl,Lithium Chloride Monohydrate Product on Alibaba.com

13 Liquids and Solids. 2 Chapter Goals 1.Kinetic-Molecular Description of Liquids and Solids 2.Intermolecular Attractions and Phase Changes The Liquid. - ppt download