Protein sequence optimization of anti-ranibizumab antibodies a, This... | Download Scientific Diagram

Comparison of Binding Characteristics and In Vitro Activities of Three Inhibitors of Vascular Endothelial Growth Factor A | Molecular Pharmaceutics

RU2646098C2 - New method of cloning, expression and purification for preparation of ranibizumab - Google Patents

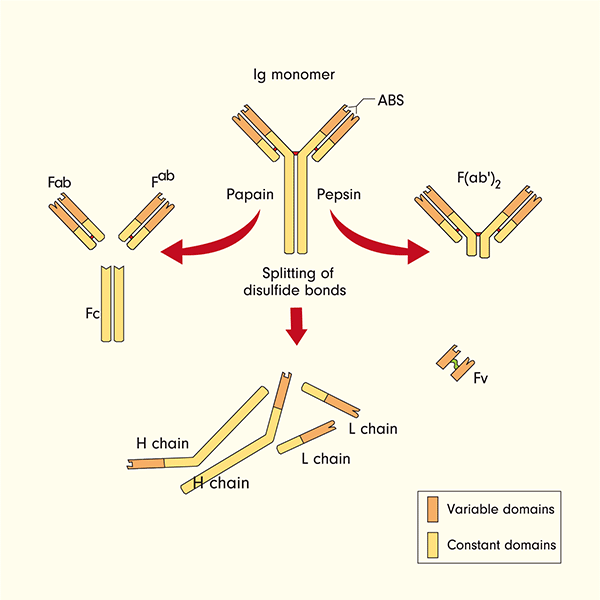
A novel strategy for efficient expression of an antibody fragment in Escherichia coli: ranibizumab as a case study - Priyanka - 2022 - Journal of Chemical Technology & Biotechnology - Wiley Online Library

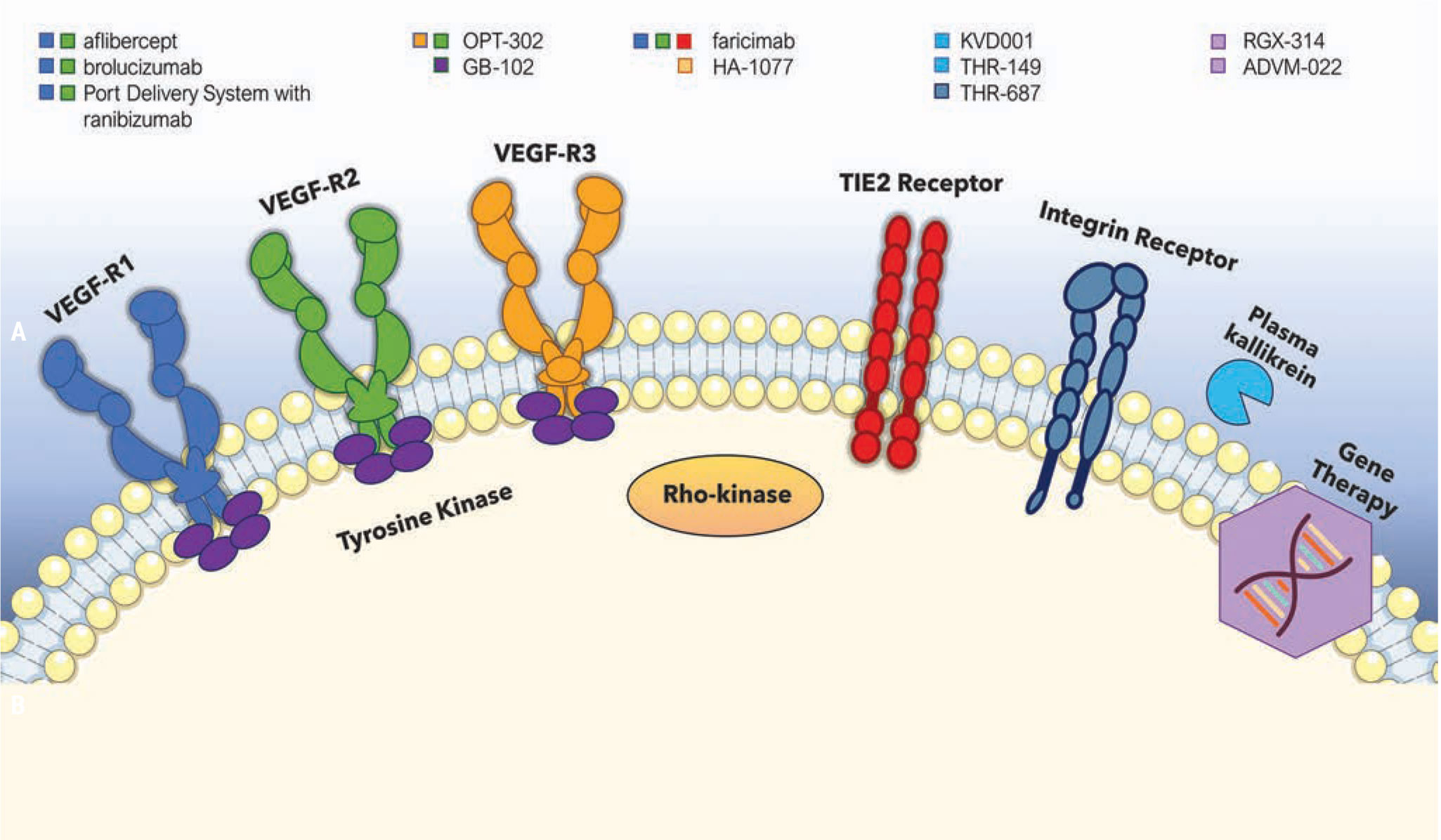
Antiangiogenic therapy for ocular diseases: Current status and challenges - Chen - 2023 - MedComm – Future Medicine - Wiley Online Library

WO2017181021A1 - Treatment of ocular diseases with fully-human post-translationally modified anti-vegf fab - Google Patents

IMGt Protein displays of the VH and V-KAPPA domains of the bevacizumab... | Download Scientific Diagram

Intraocular pharmacokinetics of intravitreal vascular endothelial growth factor-Trap in a rabbit model | Eye

Amino acid sequences of murine A4.6.1 and humanized A4.6.1 variants... | Download Scientific Diagram
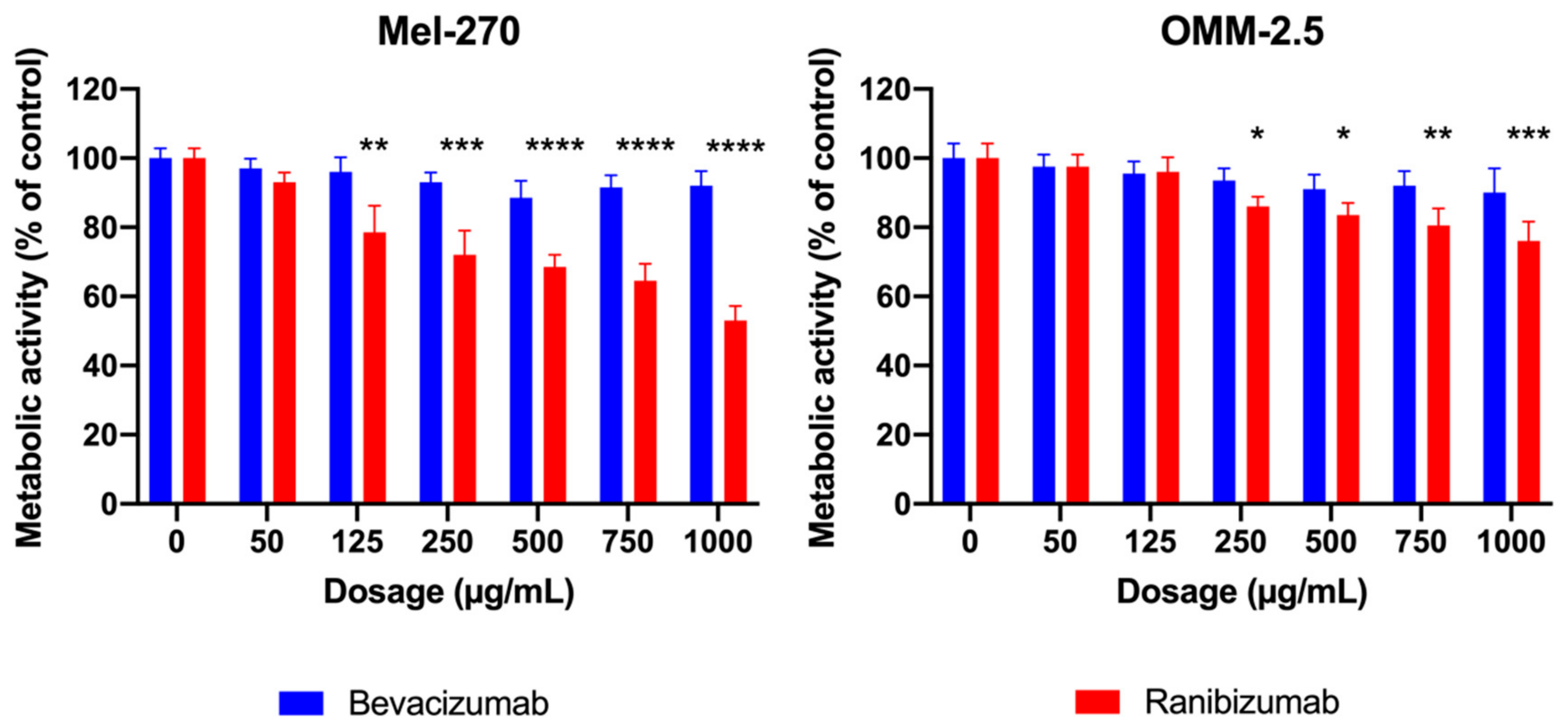
Cancers | Free Full-Text | Uptake of Ranibizumab but Not Bevacizumab into Uveal Melanoma Cells Correlates with a Sustained Decline in VEGF-A Levels and Metastatic Activities

Ranibizumab biosimilar/polyethyleneglycol-conjugated gold nanoparticles as a novel drug delivery platform for age-related macular degeneration - ScienceDirect

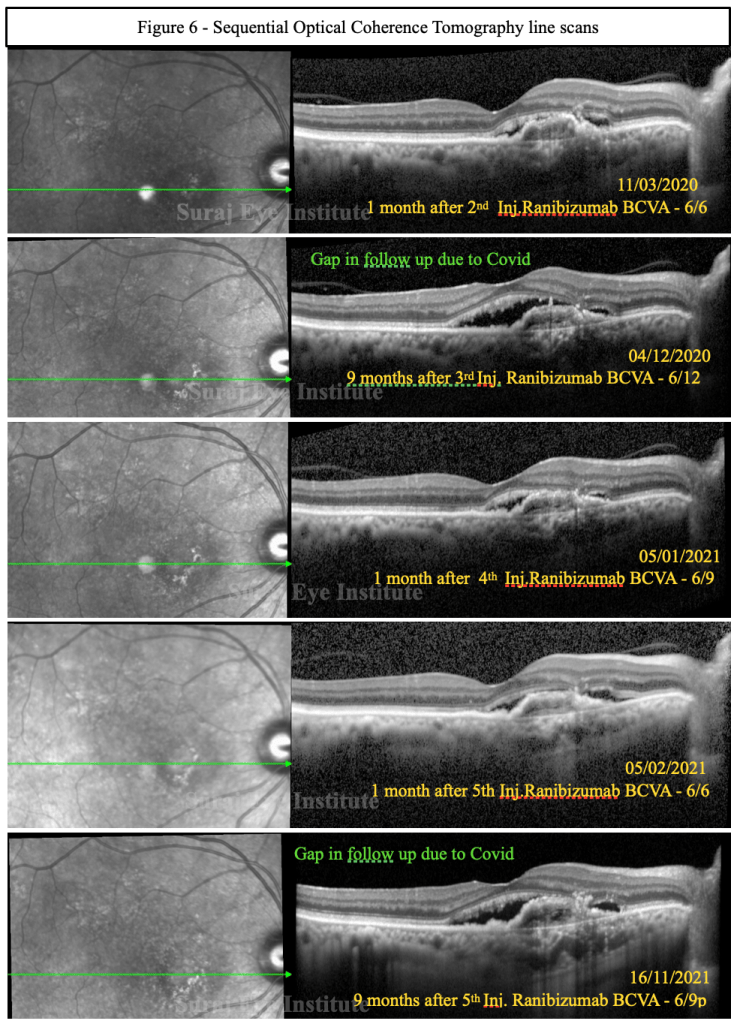
Effectiveness of Continued Ranibizumab Therapy in Neovascular Age-Related Macular Degeneration versus Switch to Aflibercept: Real World Evidence - Ophthalmology Retina

A novel strategy for efficient expression of an antibody fragment in Escherichia coli: ranibizumab as a case study - Priyanka - 2022 - Journal of Chemical Technology & Biotechnology - Wiley Online Library
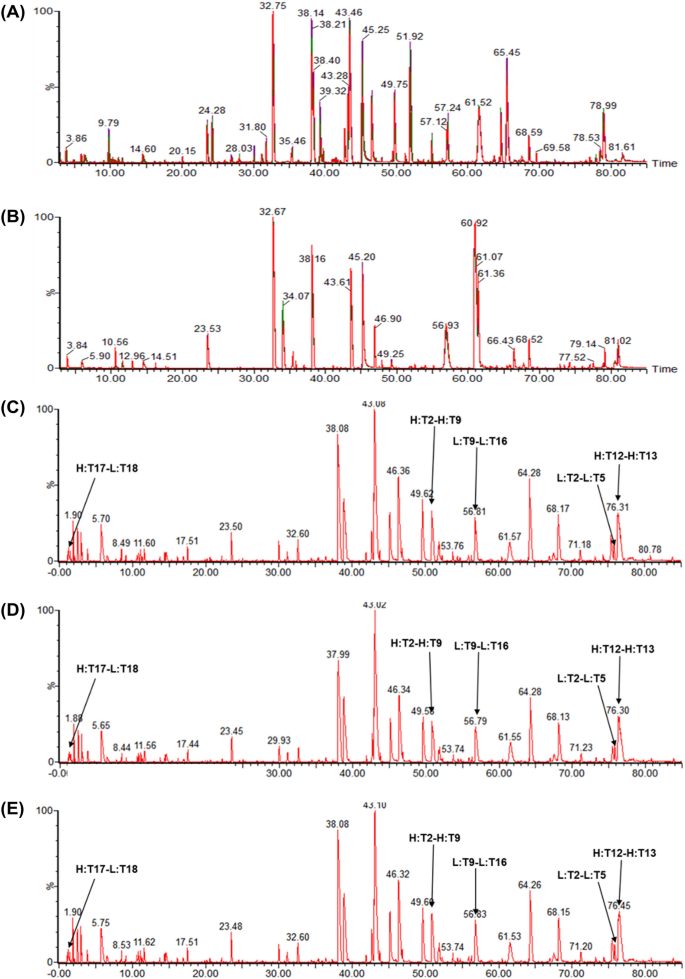
Evaluation of the Structural, Physicochemical, and Biological Characteristics of SB11, as Lucentis® (Ranibizumab) Biosimilar | SpringerLink

Efficacy and Safety of Abicipar in Neovascular Age-Related Macular Degeneration: 52-Week Results of Phase 3 Randomized Controlled Study - ScienceDirect

RU2646098C2 - New method of cloning, expression and purification for preparation of ranibizumab - Google Patents

Ocular Pharmacokinetics of Intravitreally Injected Protein Therapeutics: Comparison among Standard-of-Care Formats | Molecular Pharmaceutics