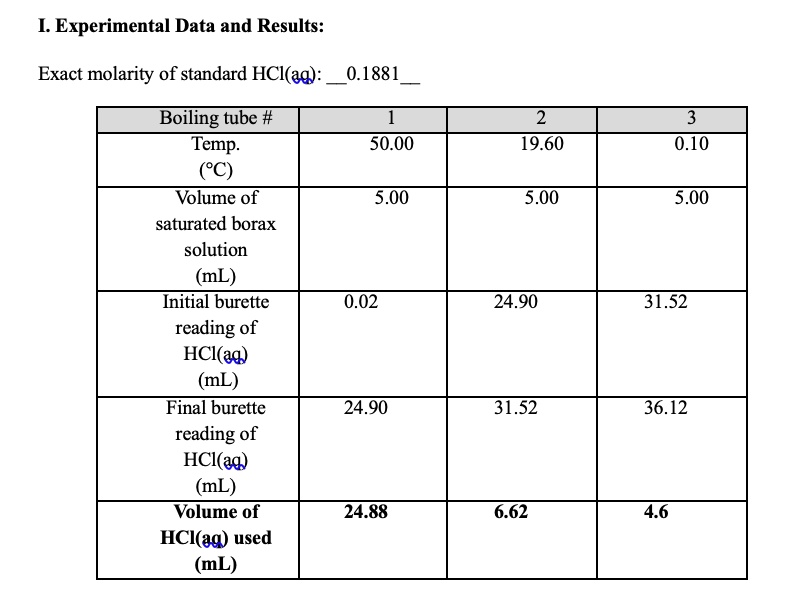
Investigating the enzyme catalysed decomposition of hydrogen peroxide by catalase effects of changing temperature, pH and concentration apparatus experimental procedure chemicals required Doc Brown's gcse biology revision study notes

SOLVED: Procedure Make sure your apparatus is gas tight and then clamp it as shown in the picture holding both the boiling tube and syringe with the correct type of clamps Place

Two flasks of equal volume connected by a narrow tube (of negligible volume) are at 300 K and contains 0.70 mole of H2 at 380 tarr . One of the flasks is